Let’s understand what is the PICO principle before we begin using it…
Evidence Based Practice (EBP) is a process and the PICO question plays an important role in that process. The question is formed based on the research question and thus does not form in a vacuum. A clear and focused foreground question should be developed instead of a background question. Only then a PICO question becomes useful to understand the impact of decisions made that affects practice.
What is the best practice? What studies are available to help with this decision?
The concepts of PICO helps us to:
- differentiate between a background and foreground question
- understand the importance of PICO to the EBP process
- identify the purpose of Interventions as a key focus of the PICO question
- understand the use of each component/element of the PICO format
- formulate clear and focused question(s) to answer the research topic
- understand the role of PICO when selecting database and formulating the search strategy
- use the PICO to select relevant studies from your search retrieval
STEPS IN EBM
| 1. Ask | Start with the patient – When talking to the patient, do you have the right questions to ask them?
Four elements of a good EBM question is the PICO
Source: http://www.cebm.net/asking-focused-questions/ Construct a relevant, answerable question derived from the case (use Clinical Question Worksheet/PICO model) |
| 2. Search | Select the appropriate resource(s) and conduct a search to find evidence to answer your PICO question
Resources refer to the type of databases (Go to NTU database webpage) |
| 3. Appraise | Read the retrieved articles from your search and ask yourself:
At the end of the appraisal, you will be able to evaluate how ‘strong’ the evidence is to address the PICO question to apply it for your patient. |
| 4. Act | Act on the evidence
Return to the patient – integrate that evidence with clinical expertise, patient preferences, and apply it to practice Evaluate your performance with this patient |
| source: | http://www.cebm.net/introduction-evidence-based-medicine/ |
Here’s a highly recommended article for you to read before you understand how PICO is applied to approach clinical research topics
Watch these short EBM videos conducted by Professor Paul Glasziou from the University of Oxford’s Centre for Evidence-Based Medicine (CEBM).
EBM video 1: Formation of PICO, PO and PO questions.
Source: [Evidence-Based-Education]. (2009, September 22). Evidence-Based Medicine in Practice – Appraisal of Clinical Trials #1. [Video File]. Retrieved from https://www.youtube.com/watch?v=QsIYwWwi_r4.
EBM video 2: Feasibility of introducing evidence-based techniques into normal medical practice.
Source: [Evidence-Based-Education]. (2009, September 17). Evidence-Based Medicine in Practice – Appraisal of Clinical Trials #2. [Video File]. Retrieved from https://www.youtube.com/watch?v=xLS1ng6D32Y&t=16s
Here are more videos on the use of PICO from CEBM
EBM Video 3: Finding the Evidence 1 – Using PICO to formulate a search question
Source: [cebmed]. (2013, January 28). Finding the evidence 2 – Turning search terms into a search strategy. [Video File]. Retrieved from https://www.youtube.com/watch?v=xLS1ng6D32Y&t=16s
What are the types of clinical questions?
Background Questions:
- Requires your general medical knowledge about the clinical condition, test or treatment
- Essentially addresses the (Who, What, Where, When, Why and How) about a disorder, test, treatment or other aspects of healthcare
- Examples:
- How does EKG work?
- What is the most common cause of stroke?
- What is SARS?
- Examples:
Foreground Questions:
- Requires specific medical knowledge to inform clinical decisions/actions in application to specific patients or problems
- Usually in Healthcare foreground questions are based on:
- therapy
- harm
- diagnosis
- prognosis
- Others include:
- clinical findings
- clinical manifestations of diseases
- prevention
- experience and meaning
- improvement
- Examples:
- Will the use of acupuncture help a smoker quit 30 years of smoking habit?
- Will the treatment of Ritalin help children who suffer ADHD?
- Among type II diabetes adults, what are the risk factors involved for those who are obese and take little exercise?
- Has 4 essential components call PICO (see below for more information)
How do you formulate answerable clinical questions?
According to the Centre for Evidence Based Medicine (CEBM) -University of Oxford,
“One of the fundamental skills required for practising EBM is the asking of well-built clinical questions. To benefit patients and clinicians, such questions need to be both directly relevant to patients’ problems and phrased in ways that direct your search to relevant and precise answers.”
Recommended database: Trip and PubMed PICO
PICO worksheets (Recommended by Medical Schools)
- CEBM (University of Oxford) – PICO Critical Appraisal Sheet (MS-Word)
- Duke University – Well Built Clinical Question (PICO) Worksheet
Use this PICO and Search Strategy Worksheet_Medlib-1xxc5qj to fill up your answers
You need to formulate well-built questions using the PICO framework. PICO stands for P=Patient, I=Intervention, C= Comparison, O=Outcome.
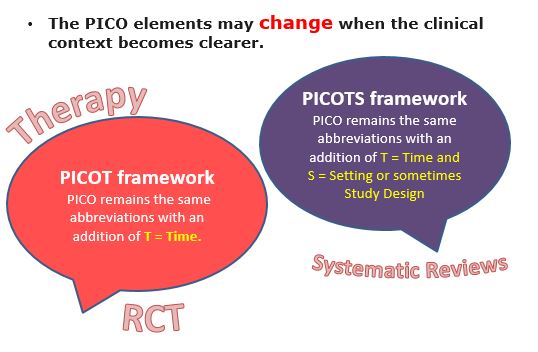
The PICO elements may change when the clinical context becomes clearer.
Example 1: For clinical scenarios addressing THERAPY/ RCTs, it's best to use the PICOT framework where PICO remains the same abbreviations with an addition of T = Time.
Here are some recommended articles on the use of the PICOT framework.
- Association between framing of the research question using the PICOT format and reporting quality of randomized controlled trials.
- What is your research question? An introduction to the PICOT format for clinicians.
- A look at the potential association between PICOT framing of a research question and the quality of reporting of analgesia RCTs.
- Framing of research question using the PICOT format in randomised controlled trials of venous ulcer disease: a protocol for a systematic survey of the literature.
Example 2: The PICOTS typology is a vital part of systematic reviews of both interventions and tests (e.g: DIAGNOSTIC ACCURACY). PICO remains the same abbreviations with an addition of T = Time and S = Setting or sometimes Study Design. The PICOTS framework is transparent and its explicit structure positively influences search methods, study selection, and data extraction.
Here are some articles about the use of the PICOTS framework. In this case, S stands for setting.
For PICOTS framework where S = study design, you need to select an appropriate type of study shown below.
- Meta-Analysis
- Description: In quantitative reviews the results from 2 or more individual quantitative studies are typically summarised using a measure of effect, which enables each study’s effect size to be statistically combined or compared in what is called a meta-analysis (Adapted from: Cook D, Murlow C, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med 1997;126:376-80)
- Systematic Review
- Description: A systematic review is a summary of the clinical literature that uses explicit methods to perform a comprehensive literature search, critically appraise and sysnthesise the world literature on a specific issue. (Adapted from Murlow C, Cook D, Davidoff F. Systematic reviews: critical links in the great chain of evidence. Ann Intern Med 1997;126:389-91)
- Randomized Controlled Trials (RCTs)
- Description: Participants are randomly allocated into an experimental group or a control group and followed over time for the variables/outcomes of interest. At the end of the trial, the effects of the different interventions are measured.
- What is n-of-1 randomised trial?
- A sub group of RCT and is conducted one only 1 participant. The participant receives 1 treatments in 1 period of time and a different treatment in another time (and this can be repeated several times). (Adapted from: Guyatt G, Keller J, Jaeschke R, et al. The n-of-1 randomised controlled trial: clinical usefulness. Our three-year experience. Ann Intern Med 1990;112:293-9)
Others include:
- Cohort Study
- Description: An observational study and also a type of longitudinal study where participants are followed over time. Participants with specific characteristics are identified as ‘cohort’.
- Case Control Study
- Case series or Case Report
- Animal Research
- In Vitro/Lab Research
- Editorials, Letters, Opinions
So can the PICO framework vary to suit the use of the research design involved in a study?
- Read this article to find out more: PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews.
To do…
Formulate a clear and focused PICO question
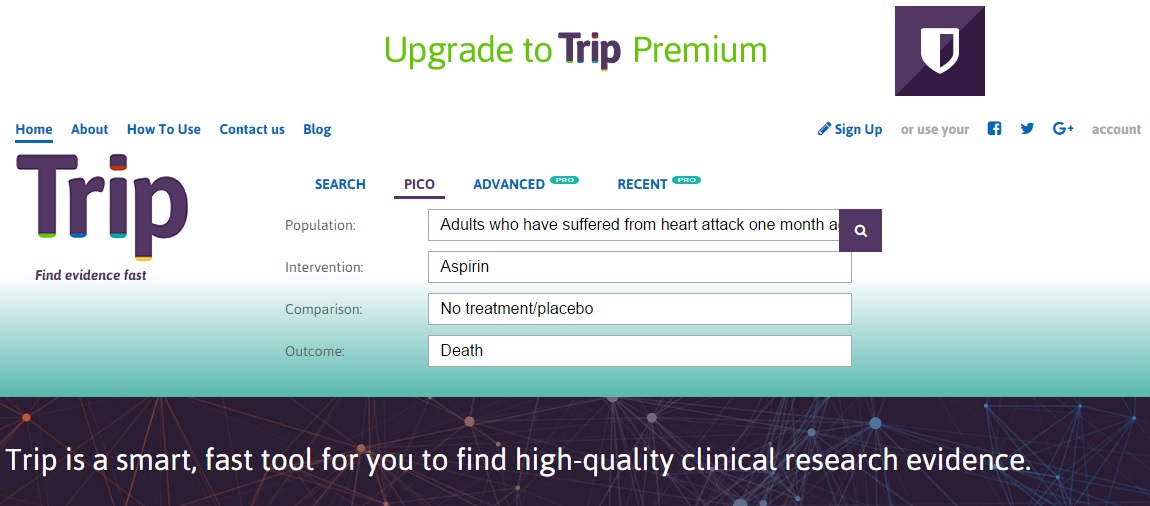
Example 1: Does aspirin reduce your risk of death after a heart attack?
- P = Adults who have suffered from heart attack one month ago
- I = Aspirin
- C = No treatment/placebo
- O= Death
- S = Systematic Review
To avoid…
Formulating a very broad question (unfocused)
Example 2: Do antibiotics help children with colds?
Formulating a very narrow question (too focused)
Example 3: Does amoxycillin reduce facial pain in teenagers (13-18) with microbiologically-proven maxillary sinusitis?
What’s next?
- What are your search terms and alternative terms identified from your PICO question?
- State your inclusion and exclusion criteria
- Example: gender, age, year of publication
- What are the irrelevant terms you want to exclude in your search?
- What databases do you want to use to plan your search?
- Example: Medline -OVID, PubMed etc.
Recommended sites for further reading from top medical schools in the world
- PICO examples from University of Oxford
- EBM – Evidence Based Medicine from Harvard University
- Explanation of the 2011 OCEBM Levels of Evidence
- Formulating Clinical Questions from Standford Medicine
Prepared by: Rebecca David, Senior Assistant Manager, Medical Library

You must be logged in to post a comment.