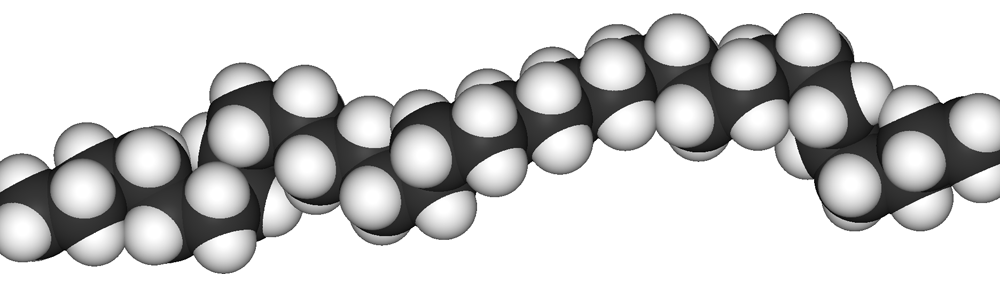
1. Chemical structure:
Polyethylene‘s chemical formula is (C2H4)n. It consists of long hydrocarbon chains ( chains of hydrogen and carbon atoms) where two hydrogen atoms are bonded to each carbon atom.
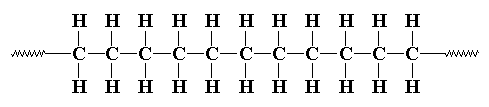
Figure 2.1 Structure of Polyethylene
2. Formation of Polyethylene
Polyethylene is formed by the process of Addition Polymerization of Ethylene, which consists of 3 steps:
a) Initiation
Ultraviolet light or heat is used to break up the weak oxygen-oxygen single bond homolytically to give 2 R-oxygen radicals, each having an unpaired electron. Radicals are unstable as they do not have a complete octet and will spontaneously attack other species to obtain a full octet.
bond homolytically to give 2 R-oxygen radicals, each having an unpaired electron. Radicals are unstable as they do not have a complete octet and will spontaneously attack other species to obtain a full octet.
The R-oxygen radical attacks the weak pi bond of the electron rich region of the alkene, forcing the pi bond to break homolytically to produce an addition radical product. This radical is also unstable, due to the incomplete octet, and goes on to react with other alkenes.
region of the alkene, forcing the pi bond to break homolytically to produce an addition radical product. This radical is also unstable, due to the incomplete octet, and goes on to react with other alkenes.
b) Propagation
The reaction of radicals with non-radicals is termed propagation. Propagation is random and it produces longer chains of alkane radicals. Different types of alkane radicals are produced too as a result of the random propagation nature. Different alkanes can then be separated.
c) Termination
Termination occurs when 2 radicals meet and combine to give a neutral stable non-radical addition product. A mixture of termination products will be formed too.
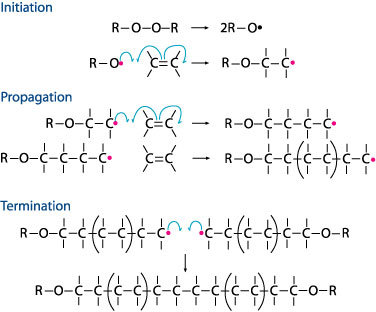
Figure 2.2 An illustration of the Addition Polymerization Process
3. Chemical properties of Polyethylene:
- High chemical resistance.
- Not easily attacked by strong acids or strong bases as there is no region of electrical charge to attract the positive proton and negative hydroxide ions. C-H bonds are non-polar and they do not react with polar and ionic reagents.
- Resistant to gentle oxidants and reducing agents as C-H bonds are generally strong and can only be oxidised under strong oxidising environment such as hot acidic potassium permanganate.
- Burns slowly as the long chain of carbons does not spontaneously oxidise in oxygen
- Crystalline samples do not dissolve at room
 temperature. It is usually dissolved at elevated temperatures with aromatic hydrocarbons or chlorinated solvents. This is due to the concept of “like dissolves like”. Crystalline alkane samples are non-polar and they only form favorable intermolecular van der waals forces of attractions with other non-polar molecules such as toluene and benzene.
temperature. It is usually dissolved at elevated temperatures with aromatic hydrocarbons or chlorinated solvents. This is due to the concept of “like dissolves like”. Crystalline alkane samples are non-polar and they only form favorable intermolecular van der waals forces of attractions with other non-polar molecules such as toluene and benzene.
4. Low Density Polyethylene and High Density Polyethylene:
a) Low-density polyethylene (LDPE)
Properties
- Density: 0.910 to 0.940 g/cm3
- Has more branching (on about 2% of the carbon atoms) than High Density Polyethylene( HDPE), thus its intermolecular forces are weaker, its tensile strength is lower, and its resilience is higher. Cross-branching increase the extent of the network of covalent bonds, strengthening the overall interaction.
- Translucent if not pigmented
- Quite flexible and tough but breakable.
- Unreactive to acids and bases
- Can withstand temperatures of 80 °C and 95 °C for a short time.
Uses
- Plastic bags
- Films
- Sheets
- Bubble wraps
- Toys
- Wire insulations

Figure 2.3 Plastic bags made from LDPE
b) High-density polyethylene (HDPE)
Properties
- Quite Similar to LDPE
- Density: 0.93 to 0.97 g/cm3
- Has little branching, giving it stronger intermolecular forces and tensile strength than LDPE.
- Harder and more opaque
Can withstand higher temperatures.
Uses
- Plastic bottles
- Buckets
- Crates
- Water pipes

Figure 2.4 Water pipes made from HDPE