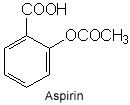
Aspirin(acetylsalicylic acid) is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and an anti-inflammatory medication.Aspirin also has an antiplatelet effect.
Aspirin is also used long-term, at low dosage, to help prevent heart attacks, strokes and blood clot formation in people at high risk of developing blood clots. Also, low doses of aspirin may be given immediately after a heart attack to reduce the risk of another cardiac arrest . Aspirin may be effective at preventing certain types of cancer, particularly colorectal cancer.
However, the main side effects of aspirin are gastrointestinal ulcers, stomach bleeding, and ringing in the ears, especially with higher doses. In children and adolescents, aspirin is not recommended for flu-like symptoms or viral illnesses because of the risk of Reye’s syndrome.
In conclusion, drugs in general should be used in the right dosage if not side effects may occur that may be lethal. By doing this project, we can know more about this common drug and consume with caution. Likewise, we can share this knowledge to the people around us and be more aware to the drug we take.
References:
Aspirin. (n.d.). Retrieved March 18, 2015, from http://www.rsc.org/learn-chemistry/resource/rws00002157/aspirin