The active ingredient of aspirin was first discovered from the bark of the willow tree in 1763 by Edward Stone of Wadham College, University of Oxford. He discovered Salicylic acid which is the active metabolite of aspirin. Aspirin was first synthesized by Felix Hoffmann, a chemist with the German company Bayer in 1897. Aspirin is one of the most widely used medications in the world, with an estimated 40,000 tonnes of it being consumed each year. Aspirin is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.
Aspirin is part of a group of medications called nonsteroidal anti-inflammatory drugs (NSAIDs), but it differs from the others in terms of the mechanisms involved. Instead of affecting more of COX-1 as compared to COX-2, aspirin inhibits enzymecyclooxygenase (COX) in an irreversible manner.
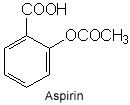
The chemical structure of aspirin:
Synthesis of Aspirin
Raw Materials:
- Phenol
- Sodium Hydroxide
- Carbon Dioxide
- Acetic Anhydride
- Hydrogen

References:
Aspirin. (n.d.). Retrieved March 18, 2015, from http://www.rsc.org/learn-chemistry/resource/rws00002157/aspirin
Reactions. (n.d.). Retrieved March 18, 2015, from http://www.aspirin-foundation.com/what/reactions.html