Introduction
What is Ozone?
Ozone is an allotrope of oxygen (physically or chemically different form of the same element) with the chemical formula O3.
What exactly is Ozone Layer?
Ozone layer refers to the concentration of ozone present in the stratosphere, which is actually very little in comparison to the total volume of gas. However, it is significantly more than the concentration of ozone present down here in the troposphere. These ozone usually helps to protect us from harmful radiation from the Sun, acting like a protective layer hence, gaining the name of ozone layer.
What are CFCs (chlorofluorocarbons)?
They are inert, non-toxic, nonflamable organic compound developed mainly as a refrigerant in rge 1800s. It replaces highly toxic ammonia or sulfur dioxide as propellants in aerosol spray cans. It is also used in styrofoam manufacture and fire-extinguishers They are good solvents for oil and grease sterilizers for surgical instruments.
When CFCs and HCFCs reach the stratosphere, the ultraviolet radiation from the sun causes them to break apart and release chlorine atoms which react with ozone, starting chemical cycles of ozone destruction that deplete the ozone layer. One chlorine atom can break apart more than 100,000 ozone molecules. The following image illustrates the process of the formation of chlorine radicals (Bauld, 2002).
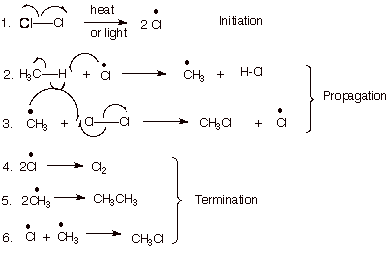
Explanation
The following process (NTU, 2015) illustrates the process of ozone destruction by chlorine radicals.
The chlorine radical that was produced is then able to attack an ozone molecule:
2Cl• + 2O3 →2ClO• + 2O2
Then two chlorine monoxide radicals combine:
2 ClO• → ClOOCl
The ClOOCl molecule then decomposes:
UV photon + ClOOCl→ClOO• + Cl•
ClOO•→Cl•+ O2
The net reaction is:
2O3 →3O2
Thus, ozone depletion is caused by the conversion of ozone molecules to oxygen molecules.
References
Bauld, N. (2002, January 1). Halogenation of methane. Retrieved January 1, 2015, from http://research.cm.utexas.edu/nbauld/ex3_key.htm
NTU, S. (2015, January 1). How CFCs destroy ozone? Retrieved January 1, 2015, from https://ntulearn.ntu.edu.sg/bbcswebdav/pid-583205-dt-content-rid-1627867_1/courses/14S2-CM8001-LEC/UNIT 2.pdf

