Introduction
Our chemical concept is about the periodic table and elements.
All matter is made up of elements or combinations of them ( compounds). Before the advent of the periodic table, the ancient Greeks believed that the world was made up of only 4 elements: Earth, Fire, Air and Water. It was only many centuries later that this belief was debunked and the modern chemical elements were discovered by various scientists, and then classified and organised into what we call the periodic table by Russian Scientist Dmitri Medeleev.
The Periodic Table
The periodic table contains both natural elements and synthetic elements. Natural elements are elements that can be found in nature. For example: Argon, Gold, and Helium. Man-made elements are elements that are manufactured by people in laboratories. Some man-made elements are: Einsteinium and plutonium.There are a total of 118 elements in all, 90 natural elements on the periodic table and 28 man-made elements.
Properties and Trends of each group
Each chemical element has its assigned atomic number, which is the number of protons in its atomic nucleus. The chemical properties of each element is determined by the number of protons and electrons in the element.
The periodic table classifies each element based on its characteristics. The periodic contains groups, which sorts them elements according to the number of valence electrons in its outer most shell, and periods, classifying the elements according to the number of quantum shells. From the periodic table, it can be clearly observed that there are 18 chemical groups, which can be further classified into the following types:
Group 1: Alkali Metals
The alkali metals consist of: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (RB), Cesium (Cs), and Francium (Fr).
- they have low melting and boiling points compared to most other metals
- they are very soft and can be cut easily with a knife
- they are characterized by their soft texture and silvery color.
- they have low densities (lithium, sodium and potassium will float on water)
- they react quickly with water, producing hydroxides and hydrogen gas
- their hydroxides and oxides dissolve in water to form alkaline solutions
- Alkali metals are among the most reactive metals. This is due in part to their larger atomic radii and low ionization energies.
- they have a valence electron configuration of ns1 and are good reducing agents (they are easily oxidized).
- these alkali metals are found naturally in nature, but not in their pure forms. Most combine with oxygen and silica to form minerals in the Earth.
Group 2: Alkali Earth Metals
The alkaline earth metals consist of beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
- they are not found freely in nature. They are present in the earth’s crust but not in their basic form.
- they have high boiling points and high melting points
- they are malleable and ductile
- they are softer and stronger than most other metals (except the alkali metals)
- they have low densities
- they have an oxidation number of +2 which makes them very reactive
- they react easily with water
Groups 3 to 12: Transition metals
- they have variable oxidation state
- they can act as homogeneous catalysts
- they form complex ion
- they form coloured compounds
- they are good conductors of heat and electricity
- they are malleable
- they are less reactive than alkali metals such as sodium
- they have high melting points except mercury which is a liquid at room temperature
- they have high densities
Group 13: Boron Family
Boron family consist of boron(B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl), and ununtrium (Uut).
Group 14: Carbon family
Carbon family consist of carbon, silicon, germanium, tin, and lead.
- (Carbon) Central element to life.
- they have nonmetallic properties.
- they forms Covalent bonds with nonmetals and ionic bonds with metals.
- Carbon is the only member of group 14 that commonly forms multiple bonds with itself.
- The elements in this group have properties changing from a non-metal, carbon to the metals tin and lead. Germanium which is in between carbon and tin shows semi metallic behaviour.
- Carbon has been known from pre-history as the charcoal resulting from partial combustion of organic matter.
Group 15: Pricogens
Pricogens consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi).
- Nitrogen is the most important component of the earth’s atmosphere (78.1% by volume).
- Nitrogen and Phosphorous are essential constituents of plant and animal tissues.
- The elements span the range from non-metallic (nitrogen and phosphorous) to the metallic (bismuth).
- Arsenic and antimony have intermediate properties and are referred to as semi-metal.
Group 16: Chalcogens
Group 16 the oxygen family consists of the elements Oxygen, Sulfur, Selenium, Tellurium and Polonium
- Oxygen, the colourless gas, necessary for life and consist about 21% of the earth’s atmosphere and sulphur is a yellow non-metallic solid.
- Selenium is not as well known, but is significant in xerography (which is a photocopying process).
- Tellurium is used in small amounts in metal alloys, tinting of glass and as catalysts in rubber industry.
- All isotopes of polonium are radioactive.
- Non-metallic characteristic is maximum in oxygen and sulphur, weaker in selenium and tellurium whereas polonium is metallic
Group 17: Halogens
Halogens consist of: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
- they are highly electronegative and reactive.
they are non-metals - they have low melting and boiling points
- down the group, the boiling point increases and volatility decreases.
- change in physical state of the halogens down the group: gas(fluorine and chlorine) to liquid(bromine) to solid(iodine)
- they are brittle when in their solid state
- they are poor conductors of heat and electricity
- they are coloured. The colour intensity increases down the group: fluorine is pale yellow, chlorine is yellowish green and bromine is red-brown. Iodine is a black solid but a purple vapour when it sublimes.
- they exist as diatomic molecules
- they act as oxidizing agents and they are reduced to halides.
- a more reactive halogen can displace the less reactive halogen from its aqueous halide ions.
Group 18: Noble gas
Noble gas consist of helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn).
- they are non-metals
- they are very unreactive gases
- they are colourless
- they exist as monotomic atoms
- they have low boiling points
General Trends of the Periodic Table
Elements show an apparent trend as we move across the period and down the group. Major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and electrical conductivity.
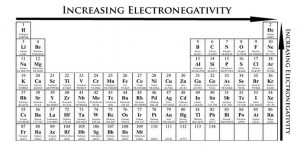
Electronegativity is the ability of an atom to attract a shared paired of electrons in a covalent bond.
Electronegativity decreases down the group. This is because as we go down the group, atomic radius increases, hence shielding effect increases as there are more electron shells. Electron shielding is the ability of an atom’s inner electrons to shield its positively-charged nucleus from its valence electrons. Hence, as shielding effect increases, there is a lesser tendency for the nucleus to attract electrons to form covalent bond.
Across the period, from left to right, electronegativity increases. This is because nuclear charge increases as there are more protons in the nucleus as we go across the period. The number of electron shells of each atom is the same hence, shielding effect remains approximately the same as electrons are added to the same outermost shell. Hence, the attraction of shared paired of electrons increases across the period.
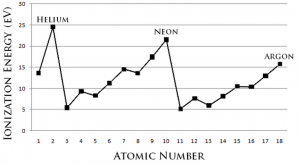
Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous state.
Ionization energy decreases down the group. This is because down the group, each successive atom has an additional shell of electrons. There is a definite increase in shielding effect as the electrons are added into another energy level. Although nuclear charge also increases, the effect of the extra electron screens far outweighs the increase in nuclear charge. Hence, the attraction between the nucleus and the outermost electrons decreases and lesser energy is required to remove an outermost electron as we go down the group.
Ionization energy increases across the period. Across the period, nuclear charge increases as number of protons in the nucleus increases. Shielding effect remains approximately the same as electrons are added to the same outermost shell. Hence, electrons are increasingly attracted to the nucleus and more energy is required to remove the outermost electron.
The noble gases possess very high ionization energies. The noble gases are chemically very stable as they have a full octet structure. They have little tendency to lose electrons and hence large amount of energy is required to remove an electron from any of the noble gases.
Atomic radius is the mean distance from the center of the nucleus to the boundary of the surrounding cloud of electrons.
Atomic radius decreases across the period. Across the period, nuclear charge increases as there are more protons in the nucleus. Shielding effect remains approximately the same as electrons are added to the same outermost shell. The effect of increasing proton number is greater than that of the increasing electron number. Therefore, the nucleus attracts the electrons more strongly, pulling the atom’s shell closer to the nucleus.
Atomic radius increases down the group. Down the group, the valence electrons occupy higher levels due to the increasing number of electron shells. As a result, the valence electrons are further away from the nucleus. Electron shielding prevents these outermost electrons from being attracted to the nucleus. Although the nucleus is more positively charged as protons increases down the group, the shielding effect is stronger and hence the nuclear attraction is weaker, causing the outermost electrons to be loosely held. Hence, atomic radius gets larger down the group.
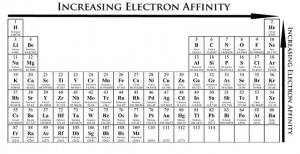
Electron affinity is the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form an anion. Electron affinity differs from electronegativity. Electron affinity defines the ability of an atom to accept an electron. Hence, an electron is transferred to the atom. On the other hand, electronegativity is the ability of an atom to attract an electron and form a covalent bond. In this case, the electron is shared.
Electron affinity decreases down the group. This is because down the group, the atomic radius increases hence an added electron is further away from the atom’s nucleus. With a larger distance between the negatively-charged electron and the positively-charged nucleus, the force of attraction is relatively weaker. Thus, electron affinity decreases.
Electron affinity increases across the period. Across the period, atoms become smaller as the forces of attraction become stronger. This causes the electron to be pulled closer to the nucleus, thus increasing the electron affinity.
The melting point is the amount of energy required to break bonds to change a substance from a solid state to a liquid state.
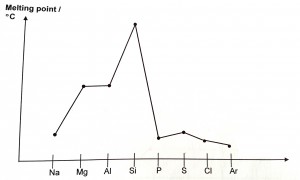
From Na to Al: They have a giant metallic structure where strong electrostatic forces of attraction exists between the cations and sea of delocalized electrons. As the number of electrons used for metallic bonding increases from 1 to 3(from Na to Al), the strength of metallic bonds increases. Hence, the amount of energy required to break the strong metallic bonds increases and thus the melting point increases.
Si: It has a giant molecular structure where strong covalent bonds exist between Si atoms in a 3-dimensional network. Large amount of energy is required to break the strong covalent bonds. Hence, the melting point is very high.
From P4 to Ar: They have a simple molecular structure where weak dispersion forces exist between molecules. Small amount of energy is required to break these forces and hence the melting points of the non-metals are generally very low. The strength of the dispersion forces depends on the number of electrons in the molecule. The more electrons present in the molecule (i.e bigger molecular mass), the stronger the dispersion forces. Hence, the variation in melting point follows the order S8>P4>Cl2>Ar, according to the number of electrons in the molecule.
Electrical conductivity in metals is a result of the movement of electrically charged particles. Metals contain a sea of delocalized electrons that are able to move throughout the metal lattice. It is these free electrons that allow metals to conduct an electric current.
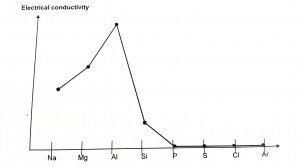
Na, Mg & Al: Giant metallic structure where cations are surrounded by delocalized, mobile electrons which are able to act as charge carriers. Hence electrical conductivities of metals are high. Number of electrons used for metallic bonding increases from 1 to 3. Hence, conductivities increase from Na to Al.
Si: It is a metalloid or semi-conductor which has properties of metals and non-metals. It has low electrical conductivity under normal conditions but conductivity increases at high temperatures or with ‘doping'(addition of other elements).
P, S, Cl & Ar: Simple molecular structure where valence electrons are localized within the covalent bonds. No mobile delocalized electrons available to act as charge carriers. They are non-conductors (insulators).
Diagonal Relationship
A close relationship is observed in certain cases between the first element of a group with the second element of the following group. This is referred to as the diagonal relationship and is observed in the following pairs. eg Li and Mg, Be and Al . When descending a group, the atomic size increases and the charge on the ion remains the same.This causes the polarizing power decreases. On moving across a period, the charge on the ion increases while the atomic size decreases, causing the polarizing power to increase. On moving diagonally the effects of size and charge partly cancel each other, so the polarizing powers are quite comparable and there is no huge change in properties. Usually, lighter elements will show such trend. The 2 elements along the diagonal also have comparable electronegativities — Li (1. 0) & Mg (1. 2); Be (1. 5) & Al (1. 5). However, diagonal similarity is much weaker than the general group similarity. This means that when we go down a group, the trend within a group is more prominent as compared to diagonal relationship trend.
References
- Nelson, P.G, Periodicity in the Formulae of Carbonyls and the Electronic Basis of the Periodic Table, Foundations of Chemistry: Philosophical, Historical and Interdisciplinary Studies of Chemistry, 15(2), 199-208. 10 p. July 2013.
- O’Gorman, J., The Elements: An Illustrated History of the Periodic Table, American Library Association, 2012; , Vol. 109 Issue 7, pp 40
- http://faculty.ksu.edu.sa/Noura_AlHokbany/222%20chem/Slidesb-C.pdf
- Inorganic Chemistry Chemistry of s and p block Elements including Noble Gases. Dr. Sushmita Chowdhury. Retrieved 06-03-2014 from: http://nsdl.niscair.res.in/jspui/bitstream/123456789/577/1/revised%20Chemistry%20of%20s%26p%20block%20elements%20including%20noble%20gases.pdf
- http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
- http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/patterns/groupsrev4.shtml