The Periodic Table
Learning the periodic table is important so as to understand the individual characteristics of commonly used elements and also learn more about their uses and applications in our daily life.
Our group will be investigating more about the trends of the periodic table and different elements play a significant role in our lives.
The elements from the periodic table have been divided into four blocks (s, p, d and f- block) elements. This classification is based on the type of the atomic orbital in which the outermost electron is located. The S orbital is the inner most orbital and it can accomodate 2 electrons. Next, the three p orbitals can each accomodate 2 electrons. Hence a total of 6 elctrons can be accomodated in the p orbitals. Thus there are two groups of s – block elements — Groups 1 and 2 whose electronic configurations are represented as [Noble gas]ns1 and [Noble gas] ns2. There are a total of 6 groups that are represented by the p orbitals. Group 13 to 18 are represented by p orbitals. In summary, the s and p block elements are called the main group elements. The remaining d and f block orbitals are represented by elements in between the main group elements.
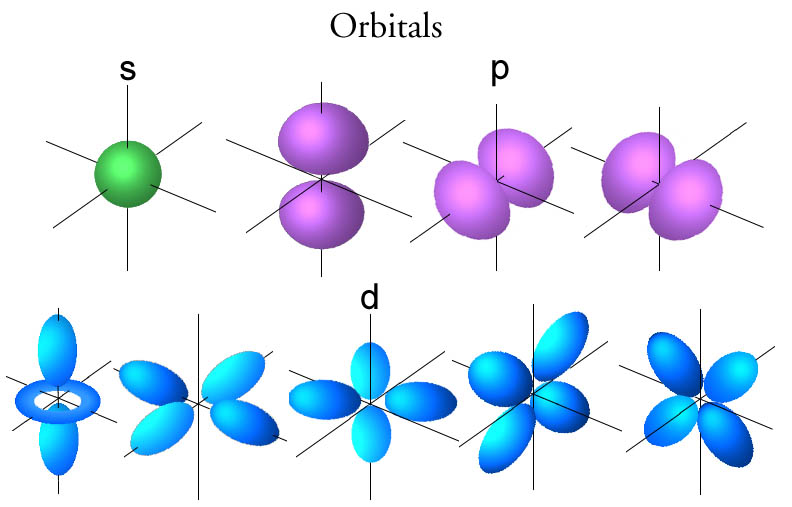
Image obtained from chemsite.lsrhs.net
