Hi everyone! We are CHEMYSTERY! For this blog, we have chosen the topic of solutions and solutes and specifically, electrolytes. The reason we choose this topic is that electrolytes are interesting and it exists on many parts of our life. From batteries that powers electronics, to blood that keeps us alive. Pretty interesting right?
Before we begin discussing about it, we need to know the basics on electrolytes. First, lets examine solutions, in which the electrolytes dissolved in, and ions, which electrolytes dissolved into.
A solution is a homogenous mixture of uniform composition and it is made up of solvents (the dissolver, present in greater amount) and solutes (the dissolved things, present in lesser amount). Some example of solutions are your everyday tea and coffee, where the tea leafs or the ground coffee is the solutes and water is the solvents.
Now what are ions?
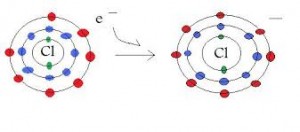
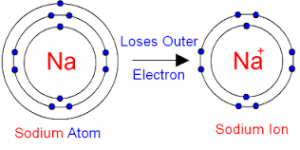
Ions are charged particles, either positive (cations) or negative (anions). The charge is caused by the addition(anion) or the removal (cation) of electrons from a neutral atom.
An electrolyte is a substance that ionizes when dissolved in suitable ionizing solvents such as water. A solution that have electrolytes ionized in it may be described as concentrated if it has a high concentration of ions, or dilute if it has a low concentration.
Do keep track of our blog if you are interested to find out more.
Thank you! 🙂