To begin with, polymers are large compounds made of very long chain molecules, each molecule made of repeated units covalently connected together called monomers. There are of two types of polymers: natural (biological) and synthetic.
To synthesise polymers, there are two ways: addition and condensation. The former, or chain growth, is that monomers form free radicals and covalently bond to each other. The latter, or step growth, is that two reacting monomers are bonded together to form a new molecule, including by-product. Polymers can be structured in linear, branched, cross-linked or networking forms.
Polymers have a wide range of uses and applications such as packing, clothes, tools, and so on. There are 600,000 synthetic polymers known today, and our group will be focus on polyethylene.
Polyethylene
Polyethylene (abbreviated PE) or polythene (IUPAC name is poly(methylene)) is among the most common thermoplastic. It has a wide application and can be found in packing products like bags, films, sheets, bubble wraps, and so on, because of its excellence properties like good strength-to-weight ratio, high corrosions resistance, cost competitiveness and ease to processing.
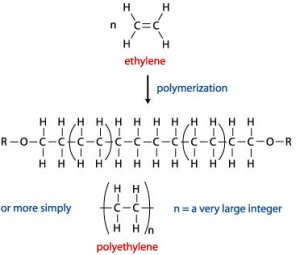
Monomers are ethylene, or ethene, a gaseous hydrocarbon with the formula C2H4. Polyethylene is produced by linking ethylene end-to-end together (addition polymerisation).
Polyethylene is classified into several different categories, based on its density and branching. Each category will have a slightly different property to serve various needs. Basically, the most important categories will be high density polyethylene (HDPE) and low density polyethylene (LDPE).
Reference:
1. http://en.wikipedia.org/wiki/Polyethylene
2. http://en.wikipedia.org/wiki/Polymer