Combustion of fossil fuels
- What are fossil fuels?
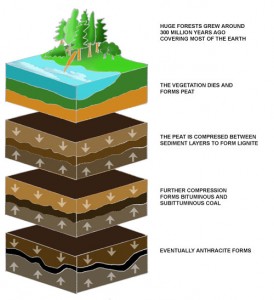
Fossil fuels are formed by dead plants and animals due to anaerobic decomposition. After millions of years, the pressure forces these compounds to become fossil fuels. Nowadays, fossil fuels are defined as hydrocarbon-containing natural resources, including coal, fuel oil or natural gas.
- Combustion of fossil fuels
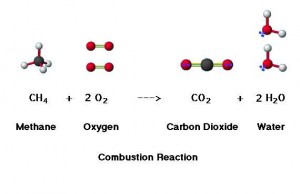
Take methane as an example. It reacts with oxygen to form carbon dioxide and water. And most importantly, it creates energy. Therefore, fossil fuels have been used as main energy resources. However, the huge amount of carbon dioxide formation leads to serious greenhouse effect, which eventually causes the global warming and a rise in temperatures around the world.
 Source: http://www.elmhurst.edu/~chm/vchembook/511natgascombust.html
Source: http://www.elmhurst.edu/~chm/vchembook/511natgascombust.html
- Deeper look into combustion
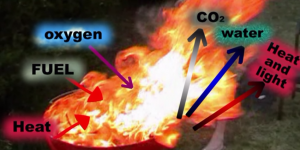
Generally, combustion is an oxidation reaction occurring at high temperatures and releases heat and light.
Source: https://www.youtube.com/watch?v=jw3ZRmKUKyA
Here is the simplest combustion reaction.
Source: http://en.wikipedia.org/wiki/Combustion
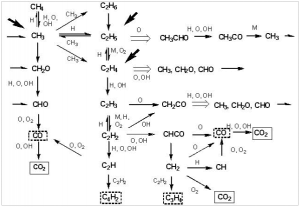
However, combustion is not that simple. It is a chain reaction in which many distinct radical intermediates participate. Combustion of hydrocarbons is thought to be initiated by hydrogen atom abstraction (not proton abstraction) from the fuel to oxygen, to give a hydroperoxide radical (HOO). This reacts further to give hydroperoxides, which break up to give hydroxyl radicals.
Source: http://www.combustion.org.uk/newsletter/newsletterjul08/warnatz.html
An interesting clip where experts analyze the repercussions of the burning of fossil fuels.