The chemical concept behind buffer solutions is fairly simple. Have a solution with components that can react with both acids and alkalis such that the addition of either does not change the pH of the solution much. The actual mechanism is a bit more complicated, and not every set of compounds can behave as a buffer. As Liam Neeson said in Taken, to be a buffer you need “a very specific set of skills”.
A buffer solution consists of a weak acid in equilibrium with with its corresponding salt (or a weak base and its salt). Take the example of the ethanoic acid/ ethanoate ion buffer system shown below,
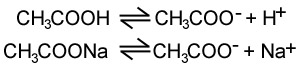
Ethanoic acid buffer system
The position of equilibrium of the first equation lies mostly on the left, while the position of equilibrium of the second equilibrium of the second equation lies mostly on the right. A simple representation is shown below.
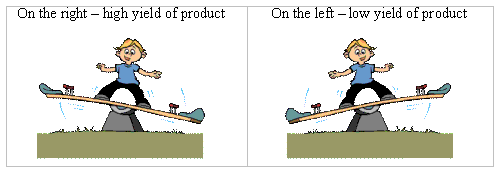
Position of equilibrium illustration
The resulting solution now has a high concentration of both ethanoic acid, as well as ethanoate ions. The ethanoic acid molecules can react with any alkali to neutralise it, while the ethanoate ions can react with any acid to neutralise them, hence keeping the pH of the solution fairly constant.
The reason this only works with weak acids is because strong acids would disassociate fully, hence the first equation would not be possible.
References:
Content (summarised from):
http://www.chemguide.co.uk/physical/acidbaseeqia/buffers.html#top
Pictures (extracted from):
http://scienceaid.co.uk/chemistry/physical/images/ethanbuffer.jpg
http://www.swotrevision.com/pages/alevel/chemistry/images/img_74.GIF
