What exactly are acids and bases?
There are three main definitions of acids and bases. Let’s explore them!
1)In the late 1800s, Swedish chemist Svante Arrhenius created the Arrhenius model in which acids are defined as proton (H+) producers in aqueous solution and bases as hydroxide (OH–) producers.
Although this model is intuitively correct, it is only limited to substances that include proton and hydroxide groups. It does not tell us why certain substances like baking soda (NaHCO3) can act as a base even though hydroxide ions are not present in it.This model was then refined by Danish scientist Johannes Nicolaus Brønsted and the Englishman Thomas Lowry to give us the second definition.


2) Under the Brønsted definition, acids and bases are defined as proton donors and acceptors, respectively.
Although the Brønsted-Lowry acid-base theory take into consideration of acids in solvents where the proton transfers do not necessarily involve hydroxide ions, it does not explain why metal ions make water more acidic.
Finally, a third concept was proposed by American chemist Gilbert Lewis which improved on the former two.
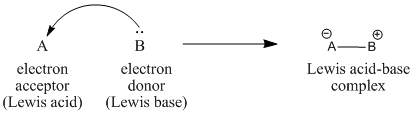
3) In Lewis’s theory, acids are electron pair acceptors and bases are electron pair donors. Acid-base reaction is defined as any chemical reaction that involved exchange of valence electron pairs to break and form bonds.
Characteristics of acid and base
Now that we have defined what are acids and bases, we shall look into some simple characteristics of them.

References:
Content:
http://www.shmoop.com/acids-bases/acid-base-definition.html
http://chemwiki.ucdavis.edu/Physical_Chemistry/Acids_and_Bases/Acid#The_Br.C3.B8nsted-Lowry_Definition
Pictures:
http://chemistry.tutorvista.com/inorganic-chemistry/arrhenius-theory.html
http://www.shmoop.com/acids-bases/acid-base-definition.html
http://www.lakelandschools.org/webpages/lburris/resources.cfm?subpage=20055
http://employees.csbsju.edu/cschaller/principles%20chem/acidity/acid%20lewisABC.htm
http://images.flatworldknowledge.com/averillfwk/averillfwk-fig04_x012.jpg

