Apart from the magical process of s-p mixing, another magical process happens during the formation of molecular orbitals – sp-hybridisation!
To summarise, there are three main types of s-p hybrids available and they are as follows:
- sp3
- sp2
- sp
The shapes and angles of the hybrid atomic orbitals formed from s-p hybridisation are summarised below.
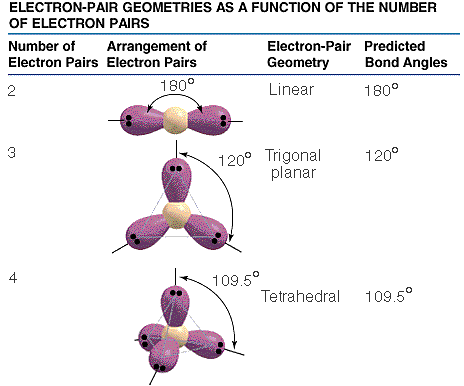
Bond angle and shapes associates with sp, sp2 and sp3 hybridisation
Sp3 hybridisation
One s orbital and three p orbitals hybridise to form four degenerate sp3 hybrid atomic orbitals (HAOs). The HAOs point towards the corners of a tetrahedron, thus, forming tetrahedral shape upon forming molecular orbitals. The bond angle in compounds with sp3 hybridisation is 109.5o, allowing the compound to be more stable.
One example of a sp3 hybridised compound is C2H6.

Atomic orbitals overlap in C2H6
Sp2 hybridisation
Sp2 hybridised compounds are planar with a bond angle of 120o. This is because three degenerate sp2 hybrid atomic orbitals are produced.
One of the sp2 hybridised compounds are CH2O.
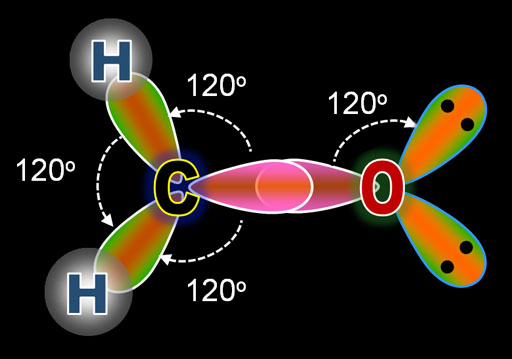
Atomic orbitals overlap in CH2O
Sp hybridisation
Sp hybridised compounds are also planar, with a bond angle of 180o that will keep bond pairs as far away from each as possible.
An example of a sp hybridised compound is a C2H2.

Atomic orbitals overlap in C2H2