The advantage of MO theory becomes more apparent when we think about π bonds, especially in those situations where two or more π bonds are able to interact with one another. Some of the delocalized molecular orbitals that result will be stabilised, while others will be destabilised.
Let us first consider the π bond in butadiene using MO theory.
From valence orbital theory we might expect that the C2-C3 bond in this molecule to be able to rotate freely since it is a sigma bond. Experimentally, however, it is found that there are significant barriers to rotation about this bond. It is also found that the entire molecule is planar. The C2-C3 bond, while longer than the C1-C2 and C3-C4 double bonds, is also significantly shorter than a typical carbon-carbon single bond.
These observations can be attributed to the concept of delocalized π bonds. The four p orbitals are all parallel to one another, and thus there is π-overlap not just between C1 and C2 and C3and C4, but between C2 and C3 as well. The 4 atomic orbitals have combined to form 4 π molecular orbitals.
In the π system, there are no degenerate MO i.e. all MO will be in different energy levels. Thus we will have 4 MOs, all with different energy levels.
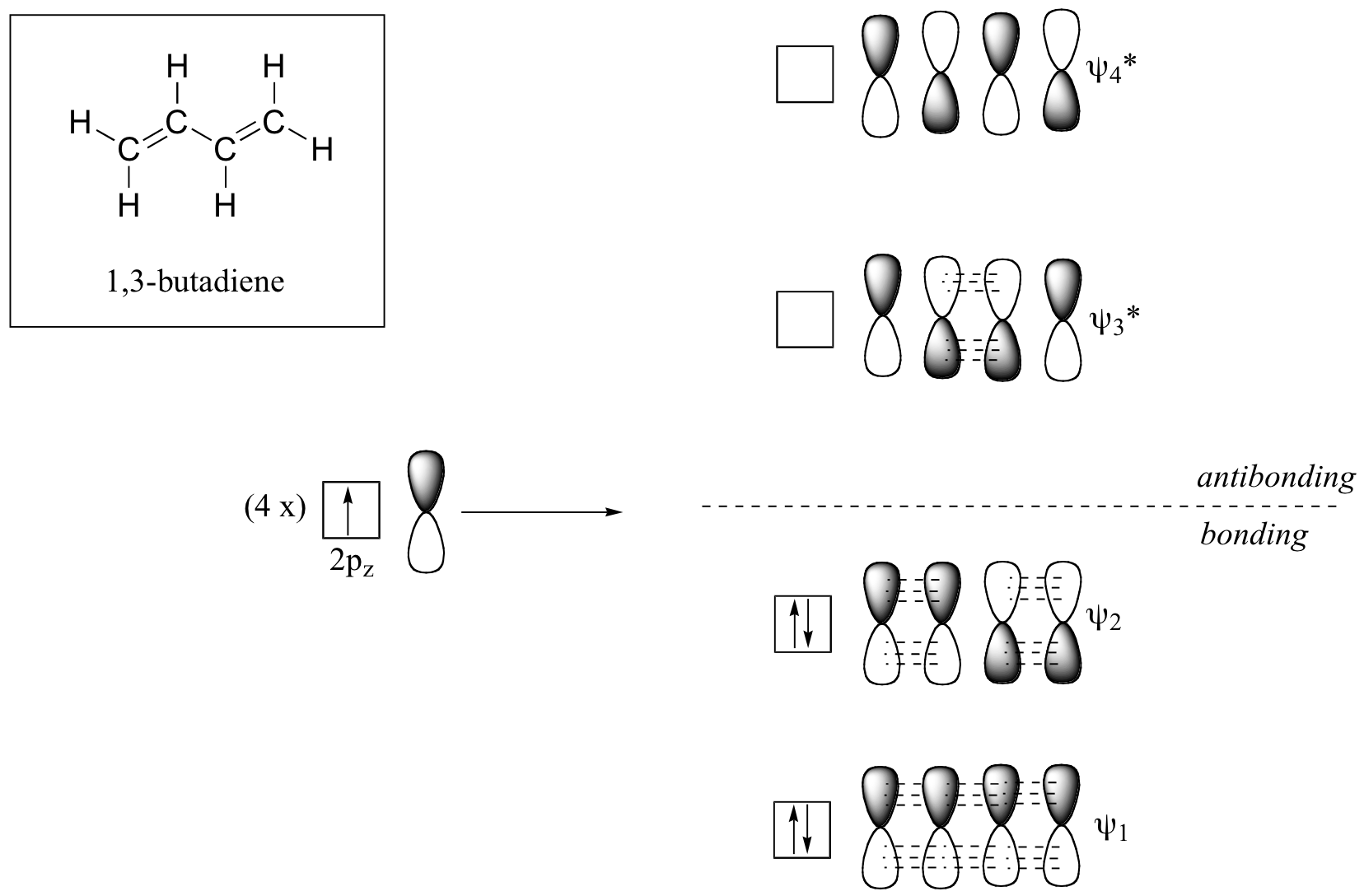
The lowest energy molecular orbital, Ψ1, has zero nodes, and is a bonding MO.
Slightly higher in energy is the Ψ2 orbital. This orbital has one node between C2 and C3, but is still a bonding orbital due to the two constructive interactions between C1-C2 and C3-C4.
The two higher energy MOs are denoted Ψ3* and Ψ4*, and are anti-bonding. The energy of both of these MOs is higher than that of the AOs of which they are composed.
Ψ3* has two nodes and one constructive interaction.
Ψ4* has three nodes and zero constructive interactions.
Filling up the MOs with regards to Aufbau’s principle, we will get 2 pairs of electrons on the bonding MOs and no electron on the anti-bonding MOs. This gives us a bond order of ½(4-0) = 2. This thus corresponds to the number of π bonds that the molecule has!
Now, you might be wondering, how on earth are you supposed to come up with the MO diagram of the π system!? Fret not, there is a trick to this!
First, we draw out evenly spaced dot representing the positions of the atom (numbered 1 to 4).

Then we add two extra atomic positions – one dot to the left of 1 and one dot the right of 4 numbered 0 and 5. There are no atoms here but these are necessary for the graphical constructions of the desired MOs.
![]()
Then, draw a half sine wave is between the positions 0 and 5.

The contribution of each AO to the MO is given by the height of the sine wave at the position of that atom.

Similarly, all the other three MOs can be deduced this way. The MO above gives the MO with lowest energy, and it has zero nodes. With increasing energy the number of nodes increase, i.e. the next MO will have 1 node, and the one after that will have 2 nodes, and so on. The MOs for the π-system is shown below:

With this method, the MOs for a π-system composed by any number of p-orbitals can be predicted. 😀
Now, to determine whether a π MO is bonding, non-bonding or anti-bonding, we have to see whether there are more constructive or destructive interactions. Let us take a look at the above example.
For 1π, all interactions are constructive, hence MO is bonding.
For 2π, 2 interactions are constructive (between positions 1 & 2 and 3 & 4) while one is destructive (between positions 2 & 3). Hence MO is bonding.
For 3π, 1 interaction is constructive (between positions 2 & 3) while two are destructive (between positions 1 & 2 and 3 & 4). Hence MO is anti-bonding.
For 4π, all interactions are destructive, hence MO is anti-bonding.
Note that when there is an odd number of MOs, the MO with equal number of constructive and destructive interactions will be non-bonding.
