Atoms have orbitals where electrons reside. A maximum of 2 electrons can occupy each orbital.
Orbitals can be found in different principal quantum shells, the table below details in the orbitals in the first 4 principal quantum shell:
In addition to being found in different principal quantum shells, the atomic orbitals are also of different shapes. For example, s orbitals are spherical and p orbitals are dumb-bell shaped while d and f orbitals have more complex shapes.
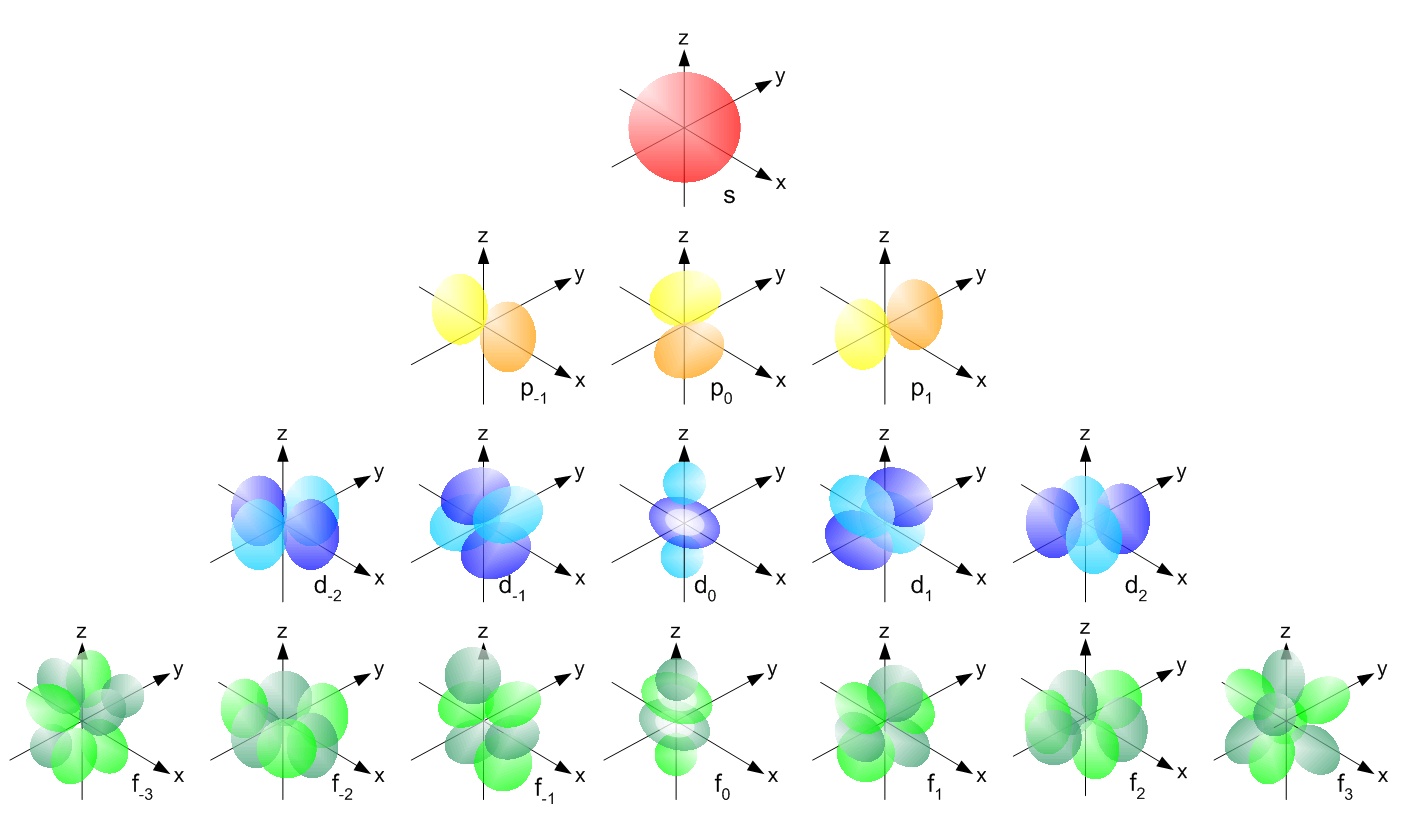
Fig 1.2 Picture showing the shapes of s, p, d, f atomic orbitals
During the formation of molecular orbitals, electrons are shared between atoms in a covalent bond. Atomic orbitals of the same symmetry are able to interact to form molecular orbitals. The number of molecular orbitals, m, in terms of the number of atomic orbitals involved in bonding, n, is as such:
m=n
Thus, if 2 atomic orbitals interact to form molecular orbitals, 2 molecular orbitals will ultimately be formed.
