My research spans molecular virology and antiviral immunology, offering a comprehensive investigation into RNA viruses and the dynamics of host innate immunity. My team and I are committed to pioneering innovation, addressing key questions in these areas:
- Dengue Virus Research: My graduate research provided groundbreaking insights by delineating the structures of the dengue virus NS3 protease-helicase in complex with ATP and RNA substrates using X-ray crystallography. These findings, crucial for understanding RNA unwinding and ATP hydrolysis in viral replication, were published in esteemed journals (Luo et al., JVI 2008; EMBOJ 2008; JBC 2010; Swarbrick et al., NAR 2017), advancing our comprehension of viral biology. We extended our research to mechanisms of viral genome maturation and host defense evasion in Dengue and Zika viruses, resulting in key publications in PLoS Pathogens, PNAS, Nature Communications, and Science. These contributions are instrumental in antiviral drug discovery for flaviviruses. Our lab is a sought-after partner for functional assays and expertise in antiviral programs, collaborating with over fifty institutions worldwide through Addgene and partnering with leading academic and pharmaceutical organizations to develop new antiviral therapies.
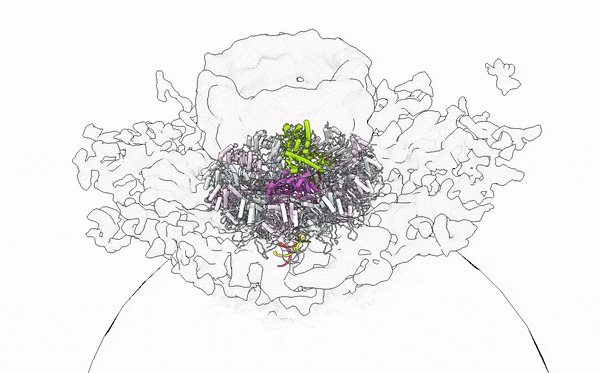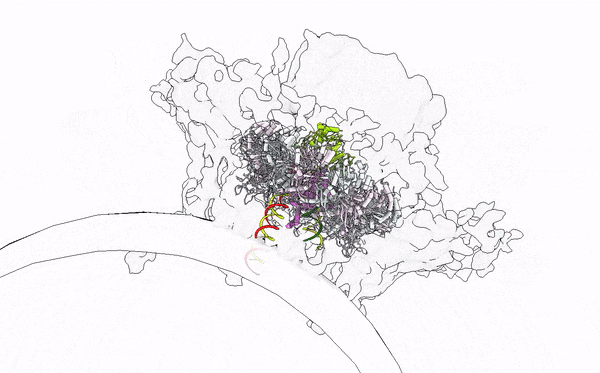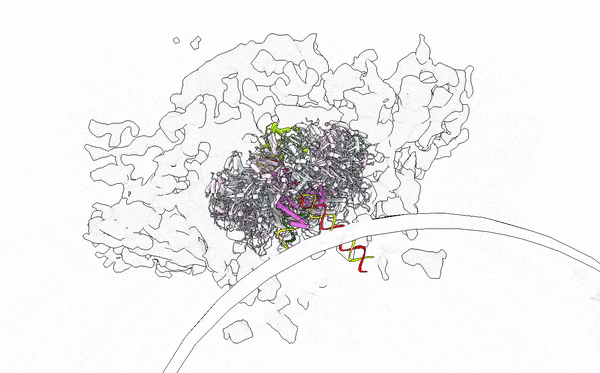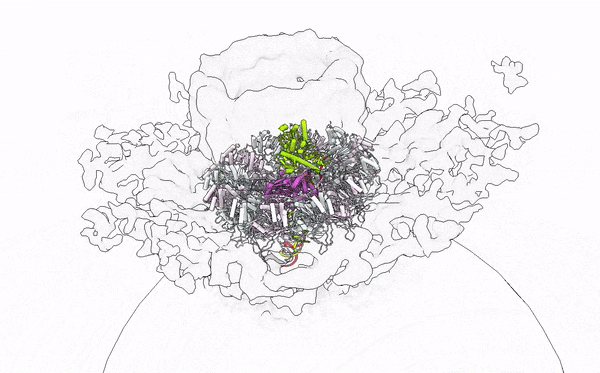
- Alphavirus Replication: Intrigued by RNA viruses’ diversity, we embarked on a detailed investigation of alphaviruses, focusing on globally concerning species like Chikungunya and Mayaro viruses. Our work has unveiled their replication mechanisms, establishing a foundation for future therapeutic developments. Publications by Law et al. (PNAS, 2019; JVI, 2021), Zhang et al. (Cell Host & Microbe, 2021), and Tan et al. (Nucleic Acids Research, 2022; Science Advances, 2022) provide a comprehensive understanding of the alphavirus replication apparatus, clarifying the chemical and structural bases of viral RNA biogenesis.
- Immune Sensing at RNA level: My tenure at Yale University was crucial for studying the interplay between viral assaults and immune defenses, focusing on RIG-I-like receptors (RLRs). Through X-ray crystallography, cryo-EM, and HDX-MS, we elucidated the RNA sensing and activation mechanisms of antiviral RLRs, significantly advancing knowledge of how viral infections are detected in humans. Our findings have led to the development of immunomodulatory RNA molecules, now in preclinical trials as vaccine adjuvants and anticancer agents (Patent App. 14/776,463; Patent App. 16/960,634; Yong et al., FEBS Letter 2019; Ho et al., JVI 2019; Peng et al., JEV 2022; SG Patent Application No. 10202202517W). In collaboration with Atomwise, Inc., we are designing small molecule immune modulators with deep learning in drug design, aiming to innovate antiviral and anticancer therapies.
Our research continues unabated, expanding to include infectious RNA viruses like dengue, Zika, and chikungunya viruses. Looking ahead, we are committed to creating an integrative understanding of viral mechanisms versus our defense strategies, translating this knowledge into therapeutic and biotechnological advancements in antiviral research.
More about our research
Funding Support $$$
- MOE Tier 1, Tier 2, Tier 3 grants
- MOH NMRC OF-IRG
- The Industry Alignment Fund – Industry Collaboration Project (IAF–ICP)
- NRF CRP
- NTU YIP
- EMBO YIP
- LKCMedicine Internal Grant
- NTU Nanyang Assistant Professorship