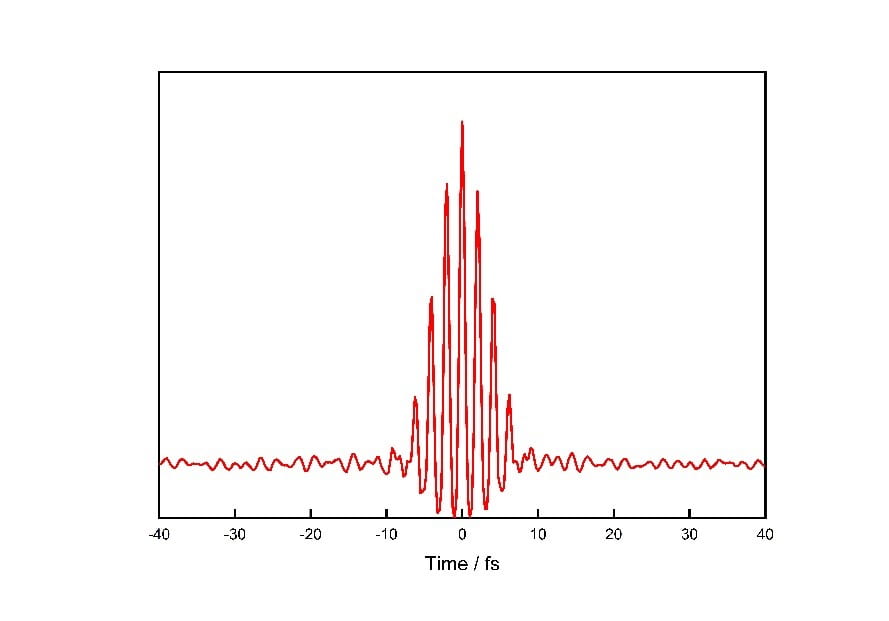
The ionization of a phenoxide ion in aqueous solution by few-femtosecond laser pulses, which produce the vibrational coherences observed. These vibrational coherences reflect the 12 vibrational frequencies associated with the phenoxyl radical.
Chemists have always been fascinated with elementary processes that happen at a molecular level such as molecular vibrations and chemical reactions. However, many of these processes occur at very short time scales and remain unknown because atoms are light and move rapidly. Hence, we need high-speed cameras to capture chemical bonds breaking and molecules vibrating which are in the timescales of femtoseconds (10-15 sec.). The ‘camera’ comes in the form of lasers that emit very brief flashes of light. The short exposure times associated with the brief laser flashes enable us to take freeze-frame snapshots of rapidly changing chemical species. This field of “Femtochemistry” led to Ahmad Zewail, being awarded the Nobel Prize in Chemistry in 1999.
In the recently published paper on Nature Communications, Assoc. Prof. Loh Zhi-Heng’s research group was able to investigate these ultrafast processes of biomolecules upon ionization in aqueous solution. Ionizing radiation ejects electrons from molecules including those that make up biological matter. It is known that excessive radiation exposure is damaging to the human body such as skin cancer. Hence, we want to study how molecules stretch, bend, and twist in response to ionizing radiation. The behavior of the molecules in response to ionizing radiation is some of the elementary processes in radiation chemistry and radiation biology, and it is therefore fundamentally important to study them. While many studies have been done in gas phase, this work was done in an aqueous medium to make it biologically more relevant.
Molecules can distort in many different ways; each way is characterized by a unique vibrational frequency, not unlike a flexible gymnast going through her routine, following the rhythm of the music. We choose to focus on phenoxide because it is closely related to tyrosine, an amino acid which plays an important role in biology shuttling electrons. In our experiment, we observe 12 vibrational frequencies, which shows that the molecule distorts in a multitude of ways in response to ionizing radiation. This is the first time that anyone has observed such rapid structural changes for an ionized molecule in an aqueous medium.
Such work provides valuable insight about the ultrafast dynamics of molecules that follow the interaction of ionizing radiation in aqueous solution. This promising line of research will extend to more large and complicated molecules, such as amino acids, as they have more degrees of freedom for atomic motion, which will make the analysis more challenging.

A snapshot of what is known as the “chirped mirror compressor” in action. It is one of the key components that was used to generate flashes of light in ultrashort duration (~0.000 000 000 000 005 s).
Read the paper on Nature Communications here.