In third place of the CoS Science Communication Writing Competition is Mah Wai Lum William from the School of Physical & Mathematical Sciences! He wrote about “A magnetic approach to cancer treatment.” Congratulations, William!
A magnetic approach to cancer treatment
Written by Mah Wai Lum William
Cancer, a disease caused by abnormal cells with unlimited growth potential, has always been the bane of humankind. It is the leading cause of death in Singapore and the second around the world. Approximately one in every 6 people will die from cancer! Unfortunately, conventional cancer treatments, i.e. chemotherapy and radiotherapy, can lead to detrimental side effects.
Chemotherapy is a drug treatment that indiscriminately kills rapid growing cells. Of course, that includes cancer cells. However, there are fast growing normal cells such as new blood cells and cells in the stomach, hair, skin and reproductive organs. Consequently, this causes side effects including appetite loss, hair loss, skin peeling, infertility and most seriously, weakened immunity. Common flu or minor wounds can become serious infections due to lowered immunity, which is akin to contracting the HIV.
Radiotherapy utilises radiation such as X-rays to kill cancer cells. These high energy radiations are considered “ionising” as they can remove electrons from atoms or molecules, thereby destroying the chemical structure of cells. As a result, not only cancer cells are damaged, normal cells that are exposed to the radiation are harmed as well. Depending on the region where the radiation is applied, the side effects vary. For example, treatment around the brain can cause blurry vision, poor memory and cognitive impairment, while treatment around the abdomen can cause vomiting, diarrhea and urinary damages.
Thankfully, cancer cells die at a lower temperature than normal cells, the former at around 42 – 45°C while the latter at around 46°C and above. This means if we heat up the cells to around 43°C, cancer cells will die while normal cells suffer minimal damage. This use of heat for cancer treatment is called hyperthermia.
One way to execute hyperthermia is to utilise magnetic nanoparticles. This is termed “magnetic hyperthermia”. By applying a magnetic field across the nanoparticles and continuously switching the direction of that magnetic field, heat is generated and used to kill cancer cells (Figure 1).
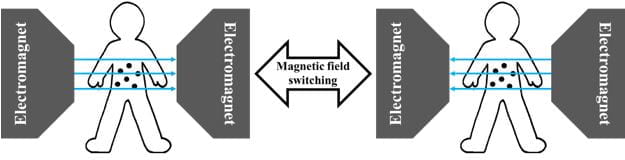
Figure 1 Application of a switching magnetic field across a region in the body with the magnetic nanoparticles
The most important property of in vivo magnetic nanoparticles is that they must posses zero remanence, i.e. when there is no applied magnetic field, they must not retain any net magnetisation. In other words, the nanoparticles cannot behave like permanent magnets. This is because permanent magnets attract one another even in the absence of applied magnetic field, and inside a body, we do not want the nanoparticles to attract one another, agglomerate into a larger particle and clog the bloodstream.
Currently, the only type of nanoparticles used for magnetic hyperthermia is called superparamagnetic nanoparticles. These nanoparticles are so small that at zero applied magnetic field, they spontaneously lose their net magnetisations due to heat from the surrounding (thus, achieving zero remanence). This is similar to demagnetising a permanent magnet by heating, except that the surrounding heat is enough to demagnetise superparamagnetic nanoparticles.
The problem with utilising superparamagnetic nanoparticles is that the fabrication process suffers a compromise between achieving particle uniformity and high reproducibility. Particle uniformity is important because different particle shapes and sizes exhibit different heating efficiencies, and to optimise the heating potential of the nanoparticles, they should all be uniform. High reproducibility allows for mass production, which is essential for widescale implementation. Consequently, the heating efficiency of superparamagnetic nanoparticles are insufficient to kills cancer cells by themselves – they need to be coupled with radiotherapy, which leads to side effects as previously discussed.
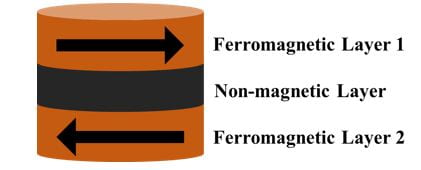
Currently, the research group of Professor S.N. Piramanayagam is exploring a new type of nanoparticles for magnetic hyperthermia – synthetic antiferromagnetic (SAF) nanoparticles. These nanoparticles consist of a non-magnetic layer (a.k.a. spacer layer) sandwiched between two ferromagnetic layers (Figure 2). How do we achieve zero magnetisation? Interestingly, by introducing a non-magnetic layer of specific material and thickness, at zero applied magnetic field, the magnetisation directions of both layers are opposite and they cancel each other out. Thus, the net magnetisation of each nanoparticle is zero, and we achieve zero remanence!
The main advantage to this novel approach is that the fabrication process does not suffer the same compromise as superparamagnetic nanoparticles. The material and thickness of each layer can be controlled for each nanoparticle, and at the same time, the production is relatively fast and cost-effective. More importantly, this offers an alternate version of magnetic hyperthermia. If successful, it can benefit mankind with a more reliable and less harmful cancer treatment.
References
[1]: Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 68(6), 394-424.
[2]: Skeel, R. T., & Khleif, S. N. (Eds.). (2011). Handbook of cancer chemotherapy. Lippincott Williams & Wilkins.
[3]: Trotti, A., Byhardt, R., Stetz, J., Gwede, C., Corn, B., Fu, K., Gunderson, L., McCormick, B., Morris, M., Rich, T., Shipley, W. & Curran, W. (2000). Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. International Journal of Radiation Oncology* Biology* Physics, 47(1), 13-47.
[4]: Perigo, E. A., Hemery, G., Sandre, O., Ortega, D., Garaio, E., Plazaola, F., & Teran, F. J. (2015). Fundamentals and advances in magnetic hyperthermia. Applied Physics Reviews, 2(4), 041302.
[5]: Koh, A. L., Hu, W., Wilson, R. J., Wang, S. X., & Sinclair, R. (2008). Preparation, structural and magnetic characterization of synthetic anti-ferromagnetic (SAF) nanoparticles. Philosophical Magazine, 88(36), 4225-4241.
[6]: Nekrashevich, I., Chang, L., & Litvinov, D. (2019). High-throughput nanomanufacturing of synthetic antiferromagnet-polymer nanoparticles with high magnetic moment, very low remanence, and high magnetic susceptibility for biomedical applications. Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena, 37(2), 022801.