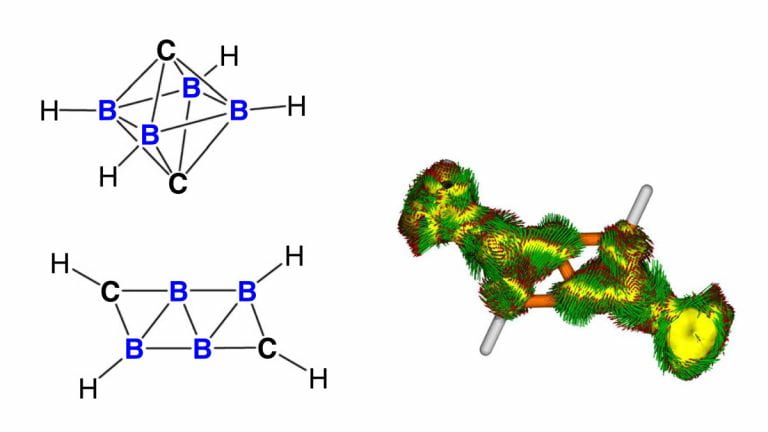
Left: Schematics of an ordinary three-dimensional carborane cluster (top) and the newly-discovered two-dimensional carborane cluster (bottom). Right: Computer simulations showing the electrons in the two-dimensional carborane cluster. Figure credit: Kinjo Rei et al.
A new form of carborane, a cluster of carbon and boron atoms arranged in an unusual flat pattern, has been isolated by chemists at Nanyang Technological University, Singapore. This discovery has overturned a 50-year old set of theoretical rules central to cluster chemistry, paving the way to a new family of chemicals.
When designing chemical compounds that can be synthesized in the laboratory, chemists are guided by a variety of well-established empirical rules. These rules predict how the shapes of molecules and atom clusters (aggregates of atoms that tend to be larger than ordinary molecules) are affected by atomic properties, such as the numbers of atoms present in the atomic orbitals.
Clusters are governed by a set of electron-counting rules known as the Wade-Mingos Rules. Originally formulated in the 1970s, these rules explain how the layout of a cluster is determined by the number of electrons in its molecular “skeleton”.
Recently, our research team has developed a new form of carborane (a cluster consisting mainly of carbon and boron) that contradicts the Wade-Mingos Rules. Unlike previously-known carborane clusters, in which the carbon and boron atoms are arranged in a three-dimensional cage-like pattern in accordance with the Wade-Mingos Rules, the newly-discovered carborane cluster has an unusually flat, sheet-like arrangement. Our findings were published in the journal Nature Communications in July 2020.
The rule-breaking existence of a flat carborane cluster had been predicted by computational chemists in 2015. However, it was not known whether such a molecule could actually be created in an actual laboratory. Carborane clusters of this sort are considered by chemists to be especially challenging to synthesize.
Our team created the novel carborane cluster, referred to as “B4C2”, via a novel chemical process based on transferring carbon atoms from an isonitrile source to a pre-formed tetra-atomic boron scaffold. X-ray spectroscopy revealed that the cluster had the sought-after flat structure. When we tested its properties, the new carborane cluster turned out to possess a remarkable level of thermally stability.
On further investigation, the B4C2 cluster revealed another surprise. Using computational simulations, we discovered that it is an aromatic compound – a type of chemical in which the atoms form an extremely stable ring. This is a violation of Hückel’s Rule, which chemists normally use to predict which molecules are aromatic based on the number of electrons in it. According to Hückel’s Rule, the B4C2 cluster is not supposed to be aromatic, but is even anti-aromatic.
This unusual feature has little precedence in the chemistry literature, so we are excited to continue investigating this class of chemicals in the future.
About the author:
Rei Kinjo is an Associate Professor in the School of Physical & Mathematical Sciences at NTU. His research focuses on the design and development of novel molecules containing p-block elements, and their applications to catalysis.

