
Associate Professor Zhi-Heng Loh (left) and his graduate student Mr Muhammad Shafiq Bin Mohd Yusof (right), shown here in their laboratory, have participated in the world’s first observation of the formation and decay of water radical ions. Photo credit: L. Kok.
An international research team jointly led by Nanyang Technological University (NTU Singapore), the US Department of Energy’s Argonne National Laboratory, and the German research centre Deutsches Elektronen-Synchrotron (DESY) has for the first time observed the ultrafast formation and breakdown of water ions created when water is exposed to ionising radiation.
The new groundbreaking observation of the H2O+ radical ion is the result of an international collaboration between NTU, Argonne National Laboratory, and DESY. The experiments were conducted at the SLAC National Accelerator Laboratory in the USA, and researchers from other institutions in the US and Europe also contributed to the study. The results were published in the journal Science in January 2020.
The lead author of the paper, NTU’s Associate Professor Loh Zhi-Heng, has studied the ultrafast dynamics of ionising molecules for over nine years. His team at NTU included PhD student Mr Muhammad Shafiq Bin Mohd Yusof and Dr Tushar Debnath, a former postdoctoral researcher who is currently a Humboldt Research Fellow at LMU Munich.
AN ION THAT PACKS A PUNCH
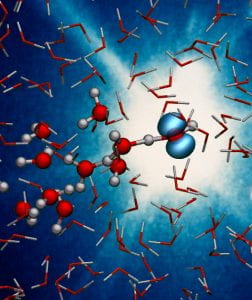
Artist’s impression of the formation of a water radical ion. Credit: Deutsches Elektronen-Synchrotron (DESY), Caroline Arnold.
When ionising radiation hits water molecules (H2O), it strips away an electron to create a water ion known as a radical cation (H2O+). This ion is highly reactive and plays a crucial role in many chemical reactions, but its incredibly short lifetime meant that it had never been directly observed, until now.
After a H2O+ radical is created, it gives up a proton to a neighbouring water molecule, creating both hydronium (H3O+) and a hydroxyl radical (OH), in a step called ‘proton transfer’. All this occurs in just 50 femtoseconds, or 50 quadrillionths of a second. Scientists believe the formation of the H2O+ radical is the first step in a cascade of reactions that eventually leads to radiation damage in biological tissue. Highly reactive radicals can strip electrons from stable compounds such as DNA, eventually causing problems such as cancer.
Proton transfer reaction is important for many fields including nuclear engineering, space travel and environmental remediation. Understanding it can help scientists determine how radiation interacts with chemicals in various aqueous environments, such as biological systems and water-cooled nuclear reactors.
NEW METHOD TO STUDY THE FASTEST CHEMICAL REACTIONS IN WATER
“While we had glimpsed the lifetime of the H2O+ radical in earlier experiments using femtosecond lasers at NTU, its direct spectroscopic observation lies beyond the capability of most tabletop equipment,” says Associate Professor Loh, who is also the Assistant Chair (Academic) at NTU’s School of Physical and Mathematical Sciences.
As he explains, the chemical reactions involving the water ion are among the fastest known to mankind. To capture its existence, the team used a state-of-the-art X-ray free-electron laser, the Linac Coherent Light Source hosted at SLAC.
”Using the ultrafast X-ray laser at SLAC enabled us to not only observe the proton transfer, but also determine what happens before, during and after the reaction,” says Associate Professor Loh.

Aeiral view of the SLAC Linac Coherent Light Source facility. Photo credit: SLAC National Accelerator Laboratory. (CC-BY-NC-SA)
Scientists have long studied the ionisation of water, with a first sighting in the 1960s by scientists at Argonne of the hydrated electron produced by the radiolysis of water. However, without a sufficiently fast X-ray probe, researchers had no way to observe the residual positively charged ion, the other half of the reaction pair.
“The truly exciting thing is that we’ve witnessed the fastest chemical reaction in ionised water, which leads to the birth of the hydroxyl radical,” said Professor Linda Young, an Argonne Distinguished Fellow and Professor of Physics at The University of Chicago, who was the senior corresponding author of the study. “The hydroxyl radical is itself of considerable importance, as it can diffuse through an organism, including our bodies, and damage virtually any macromolecule including DNA, RNA, and proteins.”
The authors suggest that understanding the formation of the hydroxyl radical could eventually help scientists devise ways to stop or reduce radiation damage in various settings, such as nuclear waste repositories or other places in need of environmental remediation.
