Projects
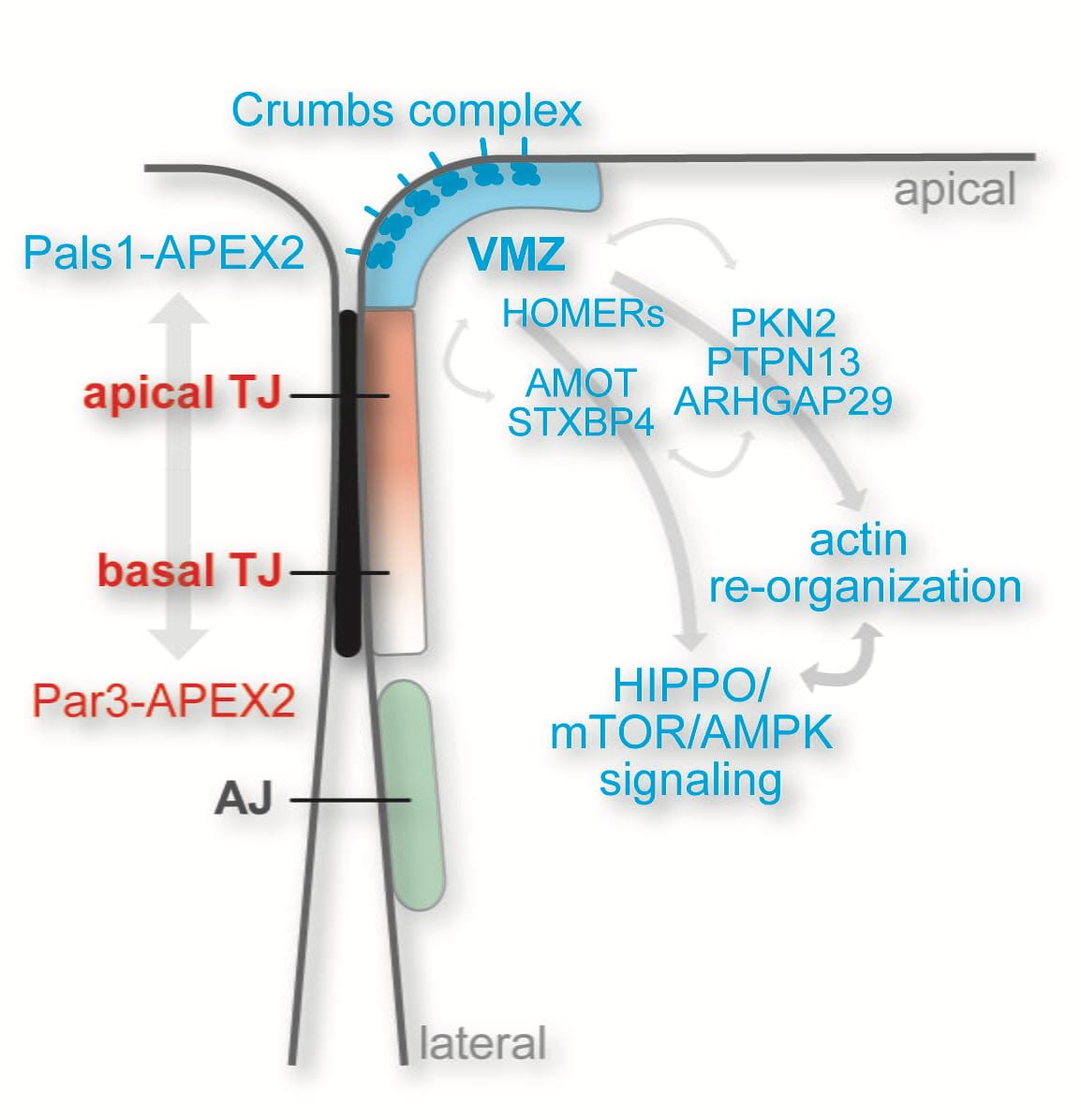
Function of the Crumbs complex in epithelial tissue morphogenesis
We have identified and molecularly defined a hitherto uncharacterized membrane domain in epithelial cells, which we call the vertebrate marginal zone (VMZ) (find out more). The VMZ is defined by the Crumbs complex, one of the three major polarity complexes in epithelial cells. We have identified a number of proteins that localize to the VMZ and that directly interact with components of the Crumbs complex. Our goal is to elucidate the function and spatio-temporal control of this VMZ-associated protein network. In particular, we are interested in understanding how the VMZ controls HIPPO signaling and actin dynamics at the apical-lateral border to regulate epithelial cell growth and tissue morphogenesis (find out more). To address this, we are applying a combination of loss- and gain-of-function approaches in 2D and 3D epithelial cultures, biochemical methods, proximity proteomics, and light and electron microscopy. In addition, we are addressing the in vivo functions of the Crumbs complex using the zebrafish as a vertebrate model organism.
Dissecting epithelial polarity development in time and space
The formation of luminal epithelial structures is tightly linked to the establishment of apico-basal polarity. De novo lumen formation, in which apical cargo is delivered to a so-called Apical Membrane Initiation Site (AMIS), relies upon the precise coordination of membrane trafficking, the assembly of cell-cell junctions, and the regulation of the cell cytoskeleton. However, how the AMIS is formed and then matures into an apical lumen is not well understood.
We have recently addressed this question using quantitative and time-resolved proximity proteomics with the peroxidase APEX2 (find out more), super-resolution microscopy, and correlative light and electron microscopy (CLEM). We find that apical cargo is transported to the AMIS via large intracellular apical precursor organelles known as “VACs” and that VAC fusion at the AMIS is temporally coordinated with the formation of tight junctions and the VMZ (find out more). Our data suggest a new model of apical domain assembly and provide important new insight into the spatio-temporal coordination of membrane trafficking and cell-cell junction assembly during epithelial cell polarity development.

Regulation of the tight junction permeability barrier
We have identified several uncharacterised tight junction-associated proteins with potential roles in signaling, membrane trafficking, and the dynamic remodelling of the actin cytoskeleton. We are particularly interested in understanding how Rho guanine nucleotide exchange factors (GEFs) and Rho GTPase activating proteins (GAPs) regulate the RhoGTPases Cdc42, Rac, and Rho to control tight junction assembly and function.
We recently demonstrated that the Rac/Cdc42 GAP Arhgap12 is recruited to tight junctions via an interaction with the scaffolding protein ZO-2. Using in vitro and cell-based assays we find that Arhgap12 suppresses branched actin assembly via N-WASP to dampen junctional tension and to promote the transport of small macromolecules across tight junctions (find out more). This work provides novel mechanistic insight into how the actin cytoskeleton controls the paracellular permeability barrier.
Moving forward we aim to dissect the molecular network that regulates the tight junction-associated actin cytoskeleton and tight junction permeability using gene editing (CRISPR/Cas9), optogenetics, live cell permeability assays, biochemical and structural approaches, and high-resolution light and electron microscopy.

Visualisation of epithelial cell architecture by correlative light and cryo electron tomography
Cell-cell junctions play fundamental roles in epithelial cells yet their native structure is unknown. We have established workflows for correlative light and cryo electron microscopy (cryoCLEM) to gain high-resolution structural information on epithelial cell architecture under close-to-native conditions. Epithelial cells grown on EM grids are vitrified, imaged by fluorescence light microscopy, and then “sliced” using cryo focused-ion-beam milling (cryo FIB-SEM) to produce thin (~200 nm) lamella suitable for transmission EM. Cellular structures exposed in such lamella are then imaged by cryo electron tomography and analysed. The EM facilities at NTU (SBS and AToM) are equipped with state-of-the-art instrumentation for cryoEM and CLEM, including a spinning disk microscope (Corrsight) dedicated for cryo fluorescence light microscopy, a cryo FIB-SEM (Aquilos), a 200 kV Arctica TEM, and a 300 kV Titan Krios TEM.