HI!! I would like to bring to your attention how your air conditioners are stabbing you on the back with the CFCs produced.
Giving you a rough idea before going into the details, CFC catalyses the decomposition of Ozone to diatomic Oxygen. Hence, the amount of Ozone decreases.
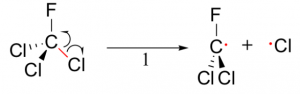
CFCs, are molecules in which all four atoms attached to one central carbon are Chlorines and Fluorines. The catalytic properties is due to the Chlorine atom present in the one carbon molecule. When “activated”, CFCs exerts their sinister reactions.
CFCs can be activated by UV light. When shined with UV, which the stratosphere has high exposure to, CFCs undergo a reaction to produce a Chlorine Radical, an atom with an unpaired electron.
This is known as the Initiation Step, which is UV-induced.
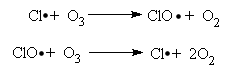
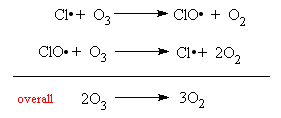
Then, the chlorine radical acts as the catalyst for the destruction of Ozone. As the radical acts as a catalyst, the radical is not consumed and is able to catalyse more ozone destruction reactions.
This is known as the Propagation Step, which can go on and on untill several millions of ozone molecules are destroyed. However, two chlorine radicals can react to terminate this propagation, hence, known as the Termination Step.
Hence the overall reaction is depicted as follow.
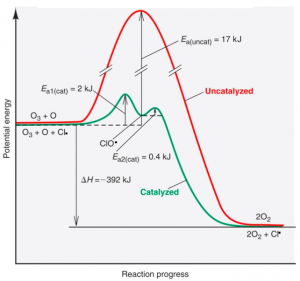
To summarise, the chlorine radical provides an alternative pathway for the degradation of Ozone. If uncatalysed, the conversion of ozone molecules to diatomic molecules requires a high input of energy (activation energy) which makes the reaction very very very slow. Below shows the two step pathway that the chlorine radical provides to reduce the energy required to kick start the reaction.
Hope you enjoyed. Thanks 🙂
References:
H. S. Rzepa (2015) ‘On the overall reaction of Ozone decomposition catalysed by chlorine radical’ Retrieved on 24 March, 2015 from http://www.ch.ic.ac.uk/rzepa/mim/environmental/html/cfc.htm
Lee Soo Ying (n.d.) CM1041 Kinetics Notes ‘On the catalysed and uncatalysed reaction energy diagram’ Retrieved on 24 March, 2015