An Introduction To Plastic
Polymers are typically macromolecules, which are derived from petrochemicals. They consist of repeating monomer units, which are strung together to form the larger macromolecule. Due to the large number of possible monomers that can make up a macromolecule, polymers are classified into a plethora of groups.
They vary in their melting points, elasticity, rigidity, tensile strength and crystallinity, among many other physical and chemical properties. For example, plastics are generally lightweight, although their weights vary significantly from each other.
Their properties are affected by a multitude of factors, some of which are their chain lengths, branching and interchain bonding.
An Insight On HDPE
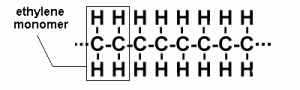
HDPEs, or High Density Polyethylenes, are macromolecules which have ethylene as their monomer.
But hold on a second, what about Low Density Polyethylenes (LDPE)? They also have ethylene as their monomers!
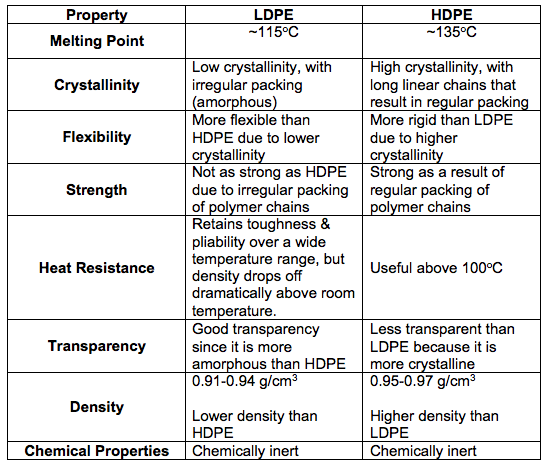
Well, HDPEs are different from LDPEs, as LDPEs are more branched. In fact, the branches on LDPEs can be up to 2 or 4 carbon atoms in length. This means that HDPEs have a greater degree of crystallinity (>90%) than LDPEs (<60%). For easier reference, a list of differences can be found below.
How Is HDPE Formed?
Petroleum is cracked to produce ethylene gas. The ethylene gas molecules are then linked together to form polymers and produce polyethylene. This process is called addition polymerisation, and is conducted under low pressure, with an aluminium-based catalyst.
Typically, the ethylene gas is diluted in a paraffin, and after polymerisation, the HDPE is recovered by cooling.
Explaining The Properties Of HDPE
As can be seen in the comparison table above, HDPE has a variety of physical and chemical properties which make it suitable for certain uses.
High Density
First up, let’s look at what gives HDPE its name! Because of the absence of branches, the long, linear macromolecules are able to be packed closely to each other. This results in a high density level.
Relatively High Melting Point
Compared to LDPE, HDPE has a relatively higher melting point. Since the macromolecules are packed close together, the intermolecular forces of attraction are much stronger than those in LDPE. This attributes a higher melting point to HDPE.
Relatively Higher Strength
HDPE is also much stronger than its low density counterpart due to the stronger bonds between the macromolecules!
Low Flexibility
HDPE is rather rigid, due to its regular, close packing, giving it a high tensile strength. This high crystallinity means that HDPE is not flexible.
Chemically Inert
Due its existence as a stable compound, and a large macromolecule, HDPE has a low tendency to chemically react. This gives it a high level of chemical resistance.
Why Isn’t HDPE Biodegradable?
HDPE plastic bag takes 1000 years to dissolve in the environment. This kind of plastic resin is a combination of toughness and stiffness with cost-effectiveness. It is well-known for its strength to density ratio, which makes HDPE a great option for manufacturers who are searching for a plastic that is resistant to breaking and cracking.
Due to its high level of strength, even in low densities, HDPE plastics are used to make a variety of durable products, including:
- Arena board
- Frames
- Ballistic plates
- Fuel tanks
- Geothermal piping systems
- Corrosion-resistant piping
- Fireworks
- Snowboard rails
- Water pipes
- Storage sheds
- Hard hats
- Folding chairs and tables
HDPE plastics are built to withstand extreme situations. Their strong and durable nature does not, however, convert easily into inert biomass through the process of biodegradation. These plastics can persist for hundreds of years, sometimes indefinitely, which contributes to the growing problem of plastic pollution.
HDPE is widely used because of its low cost, versatility and durability. This durability is partially based on plastic being an uncommon target for bacteria to decompose, which makes it a viable option for shipping items and for long-term use.
Most forms of plastic degrade in the presence of light, which is partially why the oceans are filled with tiny pieces of degraded plastic. Plastic that ends up in landfills, however, is typically buried, so it does not degrade like plastic in the ocean.
Most plastic is manufactured from petroleum the end product of a few million years of natural decay of once-living organisms. Petroleum’s main components come from lipids that were first assembled long ago in those organisms’ cells. So the question is, if petroleum-derived plastic comes from biomaterial, why doesn’t it biodegrade?

A crucial manufacturing step turns petroleum into a material unrecognized by the organisms that normally break organic matter down.
Most plastics are derived from ethylene, a simple chemical component of petroleum. When heated up in the presence of a catalyst, individual chemical units monomers of ethylene link together by forming extremely strong carbon-carbon bonds with each other. This results in polymers long chains of monomers called polyethylene.
“Nature doesn’t make things like that,” said Kenneth Peters, an organic geochemist at Stanford University, “so organisms have never seen that before.”
The organisms that decompose organic matter the ones that start turning your apple brown the instant you cut it open “have evolved over billions of years to attack certain types of bonds that are common in nature,” Peters told Life’s Little Mysteries.
“For example, they can very quickly break down polysaccharides to get sugar. They can chew up wood. But they see a polyethylene with all its carbon-carbon bonds, and they don’t normally break something like that down so there aren’t metabolic pathways to do it,” he said.
But if all you have to do to make ethylene subunits turn into polyethylene is heat them up, why doesn’t nature ever build polyethylene molecules?
According to Peters, it’s because the carbon-carbon bonds in polyethylene require too much energy to make, so nature chooses other alternatives for holding together large molecules. “It’s easier for organisms to synthesize peptide bonds than carbon-carbon bonds,” he said. Peptide bonds, which link carbon to nitrogen, are found in proteins and many other organic molecules.
Environmentalists might wonder why plastic manufacturers don’t use peptide bonds to build polymers rather than carbon-carbon bonds, so that they’ll biodegrade rather than lasting forever in a landfill. Unfortunately, while peptide bonds would produce plastics that biodegrade, they would also have a very short shelf life. “It’s an issue of ‘you can’t have your cake and eat it too,'” said Jim Coleman, chief scientist at the US Geological Survey Energy Resources Program. “When you buy a plastic jar of mayonnaise, you want [the jar] to last a few months.” You don’t want it to start decomposing before you’ve finished the mayo inside.
Peters explained that some disposable plastic products which don’t need a very long shelf life are synthesized with peptide bonds in their chemical composition. “But a carbon-carbon linkage will be more stable, so it depends on what people are trying to make.”
Engineers place HDPE water pipes and electrical conduits in the ground and expect them to last 100 years or longer. However, it is possible to add some materials to the HDPE mix that will cause it to biodegrade. Some sports bottles are purported to be made of an HDPE that will biodegrade in 1-5 years.
References:
Polymers. (n.d.). Retrieved March 11, 2015, from http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/polymers.htm
Polythene (polyethylene):Properties, Production & Uses. (n.d.). Retrieved March 11, 2015, from http://www.ausetute.com.au/polythen.htmlhttp://nzic.org.nz/ChemProcesses/polymers/10J.pdf
Biodegradable* HDPE & Resin Additive – EcoPure®. (n.d.). Retrieved March 18, 2015, from http://www.goecopure.com/biodegradable-hdpe-plastic/