2019: Loo Ying Rong Kayden, Ong Li-Jun Benjamin, Ong Si Ting Celeste
2014: Leong Kai Ting, Cassandra Ong Xian Bin, Sean Tse Mun Hin
Hi, this is is Group 2 of HG 3040.
The following is the recommended sequence of browsing for this blog. Enjoy!
1. Biological Adaption
In recent years, there has been increased interest in human language and the evolution of language. A few important questions have been raised as a result: Why have humans managed to acquire language while animals remain contented with their communication systems? Why are animals unable to acquire language even if they are taught the language?
Biological adaptation has been proposed as a possible explanation for language presence in humans and absence in animals. This chapter aims to explore language and biological adaptation in greater detail.
[Back to Table of Contents]
2. Literature Review
Language evolutionists are split over the application of the Darwinian Theory of natural selection in the explanation of language evolution. It was put forth by Pinker & Bloom (1990) that human language may have evolved by a process of biological natural selection. The human language system and its intricate cognitive requirements are explained by the development of the human brain and other organs of great complexity as a means of natural selection. Furthermore, the design features of language analysed as part of language evolution research suggest that human language is a product of natural selection, and its evolution has then enhanced humans capacity to communicate and socialise interdependently (Pinker, 2003).
The archaeological research of the Neanderthal fossils and genes also further provide evidence for a proto-language between the Neanderthal and humans with common lexical semantics (Johansson, 2013). Despite the indisputable fact that humans have language and speech while other non-human species do not, the discovery of the new DNA evidence shows a relationship between the Neanderthal and humans. This reaffirms the strong relevance of natural selection in the study of language evolution.
There are however also arguments and disagreement with the application of the Darwinian theory in the explanation of language systems used by humans and animals. Penn, Holyoak & Povinelli (2008) remarked that the differences between humans and non-humans have been downplayed, claiming that there is a lack of continuity in the approximation of complex, “systematic relational capabilities of a physical symbol system”. They also attempted to further show that this lack of continuity is also exhibited in every other domain of cognition in humans and has an intricate structure that cannot be merely explained by the prevalence of language and culture of humans. This complexity is perceived to be the difference that would mark humans from non-humans, against the Darwinian theory of gradual evolution. However, Burghardt (2008) disagrees with their argument as he finds it problematic the authors ignored the relevance of semantics in human language. Herman, Uyeyama and Pack (2008) contested the authors’ claim that the functional differences between human and non-human using the evidence of bottlenose dolphins’ relational capabilities.
In this wikichapter, Theory of Mind and functions of the FOXP2 gene are examined in relation to the explanation of language being a biological adaptation.
[Back to Table of Contents]
3. Theory of Mind (ToM)
One of the more compelling arguments proposed, is language and the theory of mind. Theory of mind (TOM) refers to the cognitive ability to represent, conceptualize and reason about mental states. In this retrospect, the ability to identify behavioral patterns or the ability to make sense of the minds of others is also considered TOM. The TOM is a trait unique to humans, though in recent studies, researchers have found that certain apes are seen to be on the brink of developing their own TOM.
As conceptual framework, TOM is the reason for the cognitive aspect of human behavior. This framework treats perceptual input as perceptual stimuli – classifying it as a belief or an intentional action warranting response. The framework further dictates appropriate response to such perceptual stimuli – in the form of inference, prediction and explanation.
Theory of Mind is not present in humans from the start – rather the cognitive framework is slowly honed as the child grows into adulthood. At different ages, there is a change in the developmental stage of TOM in humans. Miller (2006) crafted a table that outlined a timeline for the development of different aspects of theory of mind, which can be seen below.
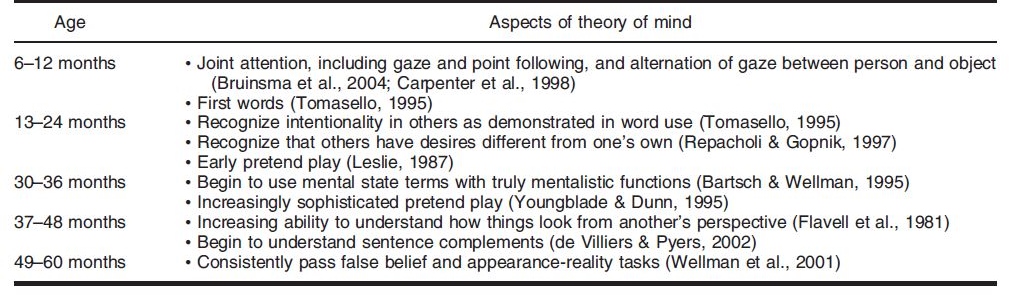
Past research has shown that there is a significant development in TOM between the ages of 3 to 4 years old. At the stage of early concept formation (between infancy to 2 years of age), there is a consistent development of TOM in children in different areas: perceptual sensitivity to goal-directed action and the ability to infer intention from human behavior. After which, the TOM sees a development in the conceptual understanding of desire and belief (between 2 to 3 years of age). The conceptual understanding of false belief is developed at 4 years of age.
The ability to understand false beliefs is commonly attributed as the significant milestone in the development of TOM. This is why there is a significant development in TOM as a child turns 4, since the child now is able to differentiate false beliefs from beliefs. There is a simple task used to test whether a child has achieved this conceptual understanding, also known as the false beliefs task.
In this task, the child is given the following scenario:A boy leaves chocolate on a shelf and then leaves the room. His mother puts it in the fridge. The child would be tasked to identify whether the boy would look first for the chocolate when he returns to the room. To pass the task, the child must understand that the boy holds the false belief that his chocolate is still on the shelf and would look there first, instead of heading for the fridge. By passing the false beliefs test, it shows that the child is able to understand that his own mental representation of the situation is different from the mental representation of another, and the child should predict the behavior of another accordingly, based on their mental representation.
It has been observed that as a child grows older, the instances in which they are able to accurately predict the behavior of another through the false beliefs test increases. At age 4, the likelihood for them to be able to identify the correct location of the chocolate would increase, compared to when they were three years old.
[Back to Table of Contents]
3.1 Language and TOM
Grammar has been assumed to be the fundamental characteristic that made language unique. Grammatical form codes speech and utterances in different basic structures – the most common being SOV, SVO or OVS. As seen in the three structures, there are different ordering to the Subject, Verb and Object agreement. Yet across the three different structures, there remains a generic structure: the agent, the recipient and the action would need to be present in each sentence regardless of the structure used.
Recent studies have shown, however, that the theory of mind has played a larger role in the evolution of language, compared to grammar. Language could not have evolved without the affiliated evolution of TOM in humans. There would be communication – even grammatical communication – but all these would simply be factual statements without any human emotion injected into the speech, somewhat like robot speech (Malle, 2004).
Miller (2006) raises some key issues regarding the complexity of the relationship between language and TOM. In order for successful communication to happen, it is essential for the speaker to have an awareness of mental states. Language is, thus, one of the key mechanisms used to express the different aspects of TOM.
Consider the following scenario:
A child points at a tree and says, “Look at that tree mummy!”
His mum asks, “What colour is the tree?”
The young boy replies, “Green!”
The above scenario shows how language can be used to express different aspects of TOM. In the very first sentence when the child points out the object, he attempt to use language to create joint attention. Here, his mother’s attention would be directed to the object that he is referring to. His mother’s reply in the second sentence is indication of this joint attention, because she acknowledges the object, and goes on further to ask him to clarify some characteristics of the object. In this sentence, the mother crafts a sentence to create communicative intent for her son to continue the conversation. The young boy’s reply in the third line is clear that the boy recognizes communicative intent presented in his mother’s question, and that is an aspect of TOM demonstrated.
As seen above, language and TOM is linked and some researchers have been concerned with identifying the possible links between the two, and they have proposed different schools of thoughts regarding the link between language and TOM.
[Back to Table of Contents]
3.2 Schools of thought
Evidently, there is some form of correlation between language and theory of mind. Drawing attention back to the table outlining the development of theory of mind in a child, it is clear that as the theory of mind is developing, the child is acquiring language at the same time (all this is under the presumption that the child is not suffering from any form of disability, like Autism or Deafness).
With this correlation in mind, many evolutionists have been fascinated with the order with which language or theory of mind came about. Was it language that preceded theory of mind, or vice versa? Or did they both co-evolve at the same time? Perhaps there may have been other factors involved in the evolution process.
For this chapter, we are concerned with two schools of thought proposed by researchers:TOM precedes language and language precedes TOM. We would then conclude and propose our own school of thought based on the findings of researchers in the previous schools of thought.
[Back to Table of Contents]
3.2.1 TOM precedes language
Malle (2004) highlights the importance of TOM, beyond the ability to understand and predict mental states of oneself and others. His stance regarding this school of thought is that “language acquisition itself appears to rely on theory of mind skills”. This argument is key in exemplifying why TOM needs to be present before any language acquisition can happen, and thus development of TOM precedes language.
Joint attention, as seen in table 1, refers to the ability to register the fact that the self and others are attending to the same object at the same time (Bruinsma et al,. 2004; Carpenter et al., 1998). Joint attention is the first aspect of TOM that is developed, when the child is between 6-12 months old. Past research has indicated the importance of joint attention in the development of TOM and the subsequent influence joint attention has on the ability for one to pick up language. The development of joint attention, would be essential for language acquisition especially in early word learning, grammatical development and referential communication.
Children suffering from autism have been known to suffer from deficits in joint attention, and that has resulted in difficulties in language learning and a subsequent deficit in their communicative abilities. In other children that may suffer from other mental handicap or general cognitive deficits, yet have developed joint attention, they still manage to acquire language much better than children suffering from autism.
[Back to Table of Contents]
3.2.2 Language precedes TOM
The strongest argument for language preceding TOM would be the approach De Villers (2001) took, that language acquisition would have a significant impact on TOM development. In order to prove this, De Villers looked at language delay, and how a delay in the acquisition of language would perhaps cause a slower rate in developing certain aspects of TOM.
To better test this theory, would be to look at the acquisition of deaf children and their TOM development. Deaf children would typically experience a delay in acquiring sign language, especially when they are born to speaking parents, that may be unable to sign with their children from day one. Studies have shown that deaf children with delayed language input would experience a delay in their reasoning of other’s mental states (de Villers, 2001). At the same time, when comparing deaf children that have delayed language input versus deaf children who acquire sign language from an earlier age (because their family members are able to provide sign input from young), it is apparent that the former is less developed in TOM compared to the latter. With regards to false beliefs, deaf children with deaf parents are not delayed in this TOM aspect, compared to deaf children with hearing parents.
While this shows how language acquisition plays an important role in the development of TOM, there is a flaw. The aspects that the researchers have been testing at this stage are mostly on how language acquisition affects false beliefs development, which is developed when the child is 49-60 months. This does not address how language precedes TOM, since delayed language input seems to affect only later stages of TOM and not the earlier stages.
Here, it appears that a possible resolution would be that TOM influenced language in the earlier years. In the later years, language would in turn affect the development of the later stages of TOM. Malle (2004) suggests that after language has been developed to a mature level, language itself becomes autonomous from TOM, and the linguistic mastery the child possesses would be used to refine TOM skills in the latter part of childhood and early adolescence.
[Back to Table of Contents]
3.2.3 Our School of thought
Having looked at both schools of thought, we have decided that TOM influences language. In order for language acquisition to happen in children, it is necessary for the children to have a theory of mind. However, at a certain age, language becomes autonomous from TOM and can overcome any form of deficits in TOM if present. Even as language becomes autonomous from TOM in the later years, TOM remains a factor that influences language.
In language acquisition, it is clear that TOM has influenced protolanguage and enabled the development of the protolanguage to language. The bulk of this chapter has focused on TOM and its influence on language, in terms of language acquisition in children. The question remains, however, how does TOM shape the evolution of language?
Beyond the area of acquisition, it appears that even in the evolution of TOM and the evolution of language, TOM evolves first, and it leads to the subsequent evolution of language from protolanguage to language. Malle’s (2004) proposed chain of model as seen below, is a model that we would advocate as well.
protolanguage → TOM → language
Malle (2004) proposes the emergence of a primitive theory of mind (TOM-1) as the first adaptive step, on which any form of language learning can be acted upon. At the primitive level of TOM-1, the features that developed at this stage are:

These features of TOM-1 is similar to the first three stages of TOM development in infants, before 12 months old. Here, Malle speculates that while these stages are developed within 12 months in acquisition, the evolution of these features of TOM-1 would have been separated by a few million years, in the hominid self.
Here, imitation and joint attention could initiate cooperative learning, while joint attention and sensitivity could improve behaviour prediction and coordinate shared activities – hunting and tool-making activities that were evident in the hominids. These three elements of TOM-1 would thus become the framework upon which protolanguage could act upon, with expressive vocalization and gestures being used at this stage to refer to certain objects or goals. At the same time, the presence of these three elements leads to communicative interactions between hominids. Hominids can imitate each other’s vocal projections through imitation, vocal reference can be reinforced through joint attention and inferential sensitivity would see hominids inferring vocal references as having some communicative intent. At the primitive stage of TOM-1, it is clear that language has yet to be developed at this stage, with gestures and vocalized expressions used to express TOM.
TOM-2 is the second stage of TOM development. As a result of TOM-1 and proto-language, there would be an increase in communication between the hominids. The simplistic nature of the protolanguage (random vocalized expressions and gestures) is likely to have caused difficulties in communication and possible miscommunication among hominids. Referring back to table 1, TOM-2 would have the same features as TOM that is developed in children between 13-24 months. TOM-2 would see the development of the ability to recognize intent expressed by others, and the ability to recognize desires others may have that differentiates from self desires.
At this stage, a more refined TOM would lead to a more developed protolanguage or PL-2. Here PL-2 would be the intermediate stage between the protolanguage and language. For clearer visualization, the development of protolanguage proposed by Malle (2004) would be:
protolanguage → PL-2 → language
At the PL-2 stage, there would be a decrease in the number of gestures used, and an increase in more precise linguistic references used, other than random vocalized expressions. Here, the presence of grammatical distinctions (nouns, verbs, adjectives) is an indication of the referential nature of PL-2 compared to the expressive nature in the previous protolanguage stage.
As observed in the discussion above, it is clear that as the different aspects of TOM are developed in hominids over the years, protolanguage is also being developed at the same time. Thus, in order for language to have developed from a protolanguage, TOM-1 and TOM-2 played essential roles in shaping the protolanguage and PL-2. Both TOM-1 and TOM-2 have seen a development of different cognitive aspects in the human brain from the time of hominids that have resulted in the subsequent development of the protolanguage to language.
Thus, the evolution of TOM is important in shaping the evolution of language from TOM.
[Back to Table of Contents]
3.3 Evaluations
It seems that the field of TOM has gaping research holes. A great deal of research has been carried out of the TOM development from infants to children aged 7 years old. After the age of 7 years, there seems to be a lack of research carried out testing TOM and language correlations. Thus it would be difficult to come to any conclusion regarding language influencing TOM, since the research thus far has been satisfied with the following conclusion – that language becomes autonomous from TOM.
Here we can look at those who were previously unable to acquire language fully because of the lack of certain aspects of TOM (children suffering from Autism). These children were still able to catch up with their peers, in terms of communication. Here, their linguistic competence would make up for their inability to understand the mental states of others, by using complex linguistic representation.
[Back to Table of Contents]
4. FOXP2
Genes are the basic building blocks for all organisms; these stretches of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) code for all functional proteins and RNA chains; these proteins and RNA chains are the essential molecules for life. These genes hold important and essential information to maintain an organism’s cells and pass on these genetic traits to their offsprings.
Natural selection is the gradual process through which biological traits become either more or less common in a population as a function of the effect of inherited traits on the differential reproductive success of organisms interacting with their environment. Natural selection is an important concept as it is responsible for shaping the genetic basis of traits, which results in biological adaptation (Pinker & Bloom, 1990).
One compelling, yet controversial, theory proposed is the Forkhead box protein P2 (FOXP2) gene and the role it possibly plays with reference to human language. The FOXP2 protein is encoded by the FOXP2 genes in humans and many studies have shown that it plays an important role, albeit unclear, in proper speech and language development.
There are a few FOXP2 studies examined in this wiki chapter, along with an evaluation and a summary of the gene’s origins.
[Back to Table of Contents]
4.1 Discovery & Origins of the FOXP2 Gene
The discovery of the FOXP2 gene can be credited to Hurst et al. who first identified an inherited defect of speech in a British family of Pakistani origin, they are known as the KE family with almost half of its members being affected by a speech disorder. There were many theories regarding the nature of the disorder with Hurst et al. (1990) suggesting that the KE family suffered from developmental verbal dyspraxia (DVD). This condition causes speech difficulty in children when saying sounds, syllables, and words due to the brain not being able to process the movement of body parts needed for speech (e.g. jaw, tongue, lips). It is known that DVD only affects speech and not the language ability of the patient. Whereas Gopnik et al. (1990) proposed the idea that the disorder afflicted by the KE family involves the impairment of the morphological component of their grammatical competence.
Video of a child diagnosed with developmental verbal dyspraxia:
4.2 Effects of the FOXP2 gene mutation
Further studies conducted discovered that on top of morphological impairment, the affected members of the KE family had impaired language processing thus causing them to have difficulties understanding speech. Furthermore, affected members of the KE family had lower IQ scores than unaffected members along with delayed development in other areas such as motor skills (e.g, walking, tying shoelaces) proving that the problem faced by the affected members are linguistic and extra-linguistic in nature. (Vargha et.al,1995). With evidence based on the inheritance pattern, it is plausible that the FOXP2 gene was the cause of the disorder affecting the KE family. However, given the diverse nature of the disorder, it is difficult to pinpoint the extent to which language was directly affected by the FOXP2 gene.
4.3 FOXP2 gene in other primates
The possibility of the FOXP2 gene having direct influences on language development in humans led to further studies focused on defining the nature and role of the gene. A team led by Wolfgang Enard compared the FOXP2 gene across different species to identify the purpose of the gene. They discovered that the version in humans differ from those of mice by 3 amino acids out of 715 and 2 amino acids when compared to chimpanzees, gorillas and rhesus macaques. Past research found that since the time when the mouse lineage split from that of the primates 130 million years ago, only 1 amino acid has changed.
This has led the researchers to the conclusion that the FOXP2 gene in humans evolved recently and rapidly due to the fact that it has developed 2 further differences from the time we, homo sapiens, diverged from the chimpanzees. Wolfgang, et.al (2002) estimated that this evolution only happened 200,000 years ago. They added that the possibility of an evolutionary advantage such as the ability to communicate verbally might have brought could have resulted in the rapid spread of the FOXP2 gene through the human population.
5. FOXP2 Gene and Vocalisations
With the possibility of the FOXP2 gene being directly related to language development, researchers aim to explore the role of this gene in the area of vocal learning. In the following section, there is the main focus on the comparison between humans and songbirds as both groups have been shown to have similar phonological and vocal learning developments. The concept of a ‘sensitive period’ is also introduced as background information to provide a better understanding of vocal development in humans and songbirds which will allow one to grasp the deeper concepts of the expression, upregulation and downregulation of the FOXP2 gene.
5.1 Sensitive Period and Vocal Learning in Humans and Songbirds

5.1.1 Sensitive Period
Both groups have a ‘sensitive period’ whereby their young have to be exposed to sounds unique to their species during their developmental stage in order to acquire them. Sound patterns learnt during these sensory learning phases are stored in their long-term memories that are afterwards useful for guiding motor production (Mori & Wada, 2015). While humans and open-ended songbirds are able to pick up new words and languages at any stage in their life, they are not able to learn vocalisations equally well throughout their lifespan.
An interesting example is how some studies done by Patricia Kuhl, a director at University of Washington’s Centre for Mind, Brain and Learning, shows that adult brains are unquestionably fixed and the process of learning new languages seems to be slower and less likely to be accent-free compared to a child’s progress (Kiester, 2001). However, only the learning curve is steeper, it is not impossible for an adult to learn a new language. Kuhl shares that it is best to learn while one’s brain is still developing, such as when one is in nursery or kindergarten (Kiester, 2001). To give a clearer illustration of human brain development, picture the correlation between one’s age and language learning. At the age of 21, it is very likely that in the process of learning a new language, one will speak with an accent that is influenced by one’s first language (Arden, 2010). On the contrary, at the age of 7, a new language that is learnt would less likely be influenced by one’s first language (Arden, 2010).
5.1.2 Vocal Learning
Songbirds are categorised into open-ended and close-ended vocal learners. Species that are commonly used in research include the Zebra finch and Bengalese finch. These finches are not able to learn new vocalisations at the adult stage so they fall under the category of close-ended vocal learners. On the other hand, the Canaries, which are open-ended vocal learners, are able to learn and imitate new songs at the adult stage (Mori & Wada, 2015).
Canaries
However, it is important to note that while there are sensitive learning periods, some mammals and birds do not need to have prior exposure in order to their species-unique vocalisations to be able to produce them. Research has shown that the FOXP2 gene could be an evolutionarily conserved gene; a gene that has not undergone any changes despite evolution (Webb & Zhang, 2004). In the beginning stages of vocal learning research, there was a bigger focus on studies related to memory and learning or sensitive periods. However, current research now leans towards genes that could be related to human language disorders, such as the FOXP2 gene (White, 2010).
Experiments have been conducted to determine if the expression of FOXP2 varies during vocal learning and the effects of the knockdown (in which gene expression is reduced) or knockout (in which genes are rendered non-functional) techniques of the FOXP2 gene. Research has shown that a knockdown or alteration of the expression of the gene affects the preciseness of birds’ song learning and impairs vocal variability (Mori & Wada, 2015). Some examples of its effects are strange omissions, repetitions or syllable ordering (White, 2010). These experimental techniques can lead to new findings and encourage further understanding of the function of a particular gene based on a comparison with other organisms that possess normal functional genes. Here, we can see that the FOXP2 possibly interferes with the process of vocal learning in songbirds, which could potentially affect vocalisations in humans. The abnormality of song learning by these birds have also been compared to the learning of words by children afflicted with DVD (Haesler, Rochefort, Georgi, Licznerski, Osten & Scharff, 2007).
[Back to Table of Contents]
5.2 Pattern of Expression
The following questions below will facilitate one’s thought process through this section:
- In the brains of vocal-learning versus non-vocal learning bird species, is the FOXP2 gene differentially expressed?
- How can the FOXP2 gene expression be compared between birds and humans
5.2.1 Avian Vocal learners vs Non-learners
Songbirds are used as a model for speech development in humans, which perhaps, could be linked to their similarities in phonological development. The parallels of songbirds and humans, which include their features of neural circuits and vocal behaviour specialised for vocal learning, allow us to understand the neural basis of vocal learning in humans (Mori & Wada, 2015). Vocal learning refers to the ability to produce new vocalisations, adapt and modify acoustic sounds and acquire novel sounds through imitation. This hypothesis is also a result of the fact that, in birds and mammals, the FOXP2’s pattern of expression in neural tissue is rather similar (Webb & Zhang, 2004). The pattern of expression of genes is a process by which the genetic code of a gene is used to direct protein synthesis and produce the structures of the cell (University of Leicester, n.d.).
For a clearer visualisation on the pattern of expression of a gene, please refer to Figure A below. The diagram shows the various pattern of expressions of the FOXP2 gene, which is represented by the pink dot, in the vocalisation center of birds’ brains. The green area is the striatum, one of the principal parts of the basal ganglia. The FOXP2 gene is often expressed in vocal-learning species such as the zebra finch (A) and the black-capped chickadee (B), it’s expression is represented by the pink dot. In non-vocal-learning species such as the ringdove (C), there is no expression of the FOXP2 gene at all and thus there is no appearance of a pink dot.
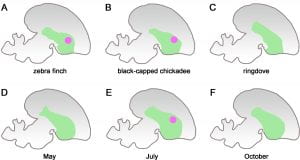
[Back to Table of Contents]
5.3 Upregulation and Downregulation
In certain types of bird species, the FOXP2 gene is said to possibly play an important role in vocalisation and it is upregulated or downregulated in neurodevelopment in the vocalisation center of vocal-learning species. Downregulation refers to a process by which the amount of cellular components such as protein or RNA is decreased in the cell. This change in quantity is usually a result of external factors. On the other hand, an increase in the cellular component is called upregulation (Merriam Webster, n.d.).
In Canaries, researchers have found that it is upregulated during song development, or more sensitive periods, and allows for them to imitate and learn bird songs (Caroll, 2005). The upregulation of FOXP2 in Canaries is especially relevant as its expression varies season to season. On the other hand, it is downregulated in adult Zebra Finches when they are singing. In non-learning species such as the ringdove, the FOXP2 is not upregulated at all (Caroll, 2005).
Research on the parallels of the FOXP2 gene between humans and non-human vocal learners is still ongoing and inconclusive. However, it has been shown that while the FOXP2 gene is likely to be not unique to just humans or homo sapiens, there could still be a possibility for the relevance of the FOXP2 gene to the development of speech and language proficiency in humans (Webb & Zhang, 2004). In essence, the FOXP2 gene is part of a pathway consisting of many other genes and it may be too early to label it as a ‘gene for speech’ (Marcus & Fisher, 2003). Nevertheless, this may be a good direction for future research – to start researching the relevant neural pathways and genes to the production of speech and language in humans.
[Back to Table of Contents]
5.4 Case study: Song Development Seasons
By using the case of song development in avians, one is able to better see how the FOXP2 is expressed during sensitive periods and real-life growth development. During song development, the FOXP2 gene is found to be expressed and upregulated. This change allows the birds to imitate and learn bird songs. In female zebra finches, there is no observation of differential FOXP2 expression in Area X as compared to its male counterparts as female zebra finches do not learn songs. Across the various four seasons in a year from July to September, Area X would prominently express more FOXP2 than the surrounding regions in the Canaries’ brains. These 3 months are when birds undergo song development and songs tend to be more plastic. They add new syllables into their songs and this increases the variability of their songs, constantly improving on their songs from the preceding year. Here, one can see that the concept of a sensitive period for acquiring new songs seems to play an important role in conjunction with song development. On the other hand, during breeding season from April to June, there is no significant expression of the FOXP2 gene as compared to the non-breeding season.
Young zebra finches that are undergoing song development and imitation and the remodelling of songs by adult canaries are great examples that researchers have found to reveal the expression of FOXP2 gene. These findings do seem to support that FOXP2 expression is only recorded to be significant in avians which are vocal-learning species who have Area X present in their brains, which propose purported links of the FOXP2 gene and vocalisation.
[Back to Table of Contents]
5.5 Evaluations
The FOXP2 gene is still continuously used to examine the history of language evolution through the understanding and analysis of language impairments. That said, there remain two issues unresolved; that is, the link between FOXP2 and human languages is still inconclusive.
There is also a lack of complete understanding of the FOXP2, and its function, especially pertaining to the acquisition and management of human languages since there is a reliance on other animals’ phonological developments to learn more about humans’. FOXP2, being a transcription gene, activates a number of other genes instead of operating within a structure of its own, also makes it difficult to attribute its effects on other genes, and its own functions to the human language. Therefore, even when it is popular to sell the theory that the FOXP2 can potentially be linked to the human-ape differences and not just language evolution, it is still commonly agreed that until it can be clearly established how the FOXP2 can be turned on and off, the claim the FOXP2 has direct links with language is still debatable (Bickerton, 2007).
[Back to Table of Contents]
6. Microcephalin and ASPM
The genes microcephalin (MCPH1) and ASPM (Abnormal spindle-like microcephaly-associated protein) are essential for proper brain growth. Microcephalin is involved in regulating the cell-cycle, especially in relation to DNA repair before cell division. ASPM helps to align the mitotic spindles in the cell so that it divides symmetrically. When non-functioning mutations occur in these two genes, a developmental defect known as microcephaly occurs in the infant. This defect results in a reduced head and brain size, often causing intellectual disability, poor motor skills and poor linguistic skills, among other symptoms.
6.1 Origin of Microcephalin & ASPM
A form of Microcephalin called haplogroup D first appeared anywhere from 14,000 and 60,000 years ago, and has since become the most common form of microcephalin throughout the world, with the exception of Sub-Saharan Africa. A new allele of ASPM first appeared sometime within the last 14,100 years, with roughly 50% frequency in Middle-East and Europe populations, less frequency in East Asia and low frequencies among Sub-Saharan African populations (Evans PD et al, 2005).
[Back to Table of Contents]
6.2 Microcephalin, ASPM and Tonal Languages
6.2.1 Introduction
Microcephalin and ASPM came to public consciousness when a paper was published in 2007 by Dan Dediu and Robert Ladd, hypothesising that the more recent variants of ASPM and microcephalin (ASPM-D & MCPH-D) are correlated with speaking non-tonal languages.
Tonal languages are languages that have variances in pitch to inflect lexical or grammatical meaning. Chinese, for example is a tonal language. Non-tonal languages such as English use pitch only at the sentence level to convey emphasis and emotion.
According to Dediu & Ladd (2007), both ASPM-D & MCPH-D showed signs of positive selection and a marked geographic structure: the areas of the world where these alleles are relatively rare also tend to be the regions where tonal languages are heavily prevalent. Statistical tests controlling for geographical and historical factors suggest a significant negative correlation between tonal languages and both ASPM-D & MCPH-D. The paper proposed that this correlation is potentially causal in nature, that the genetic structure of a population can exert an influence on the language(s) spoken by that population.
6.2.2 Criticism
However, this paper has also received much criticism for its controversial claims. Alternative models (Yu et al. 2007, Currat et al. 2006) suggest that it is possible for these variants of microcephalin and ASPM to arise without recourse to selection, which would negate the conclusion.
Below are a series of further rebuttals from Diller & Cann (2011).
Firstly, it is argued that the size of the brain is not important for language. As mentioned earlier, defective versions of microcephalin and ASPM result in microcephaly. While a microcephalic has a smaller brain, the structure of the smaller brain is largely similar to the normal human brain. Microcephalics can learn language, at least to the 6-year-old level, despite their chimpanzee-sized brain. Thus, if the size of the brain is not the key to language, then microcephalin and ASPM are likely not tied to tonal languages as hypothesised.
Another problem is that Dediu & Ladd excluded too much data from their analysis. Africa has an estimated 2092 indigenous languages, but there are only 11 in their analysis. The genetic sampling from each language population is very low. The typical language group analysed had only 19 people giving genetic information; a third of the groups had between 7 and 10 people in the sample. This statistical base, it is argued, is not adequate to test Dediu & Ladd’s hypothesis.
A third statistical problem is that the ASPM-D mutation occurred very recently, an estimated 5,800 years ago, but two-thirds of Dediu & Ladd’s samples have an occurrence of this variant of less than 30%, and only one sample is significantly over 50%. It is argued that such a small proportion of the population is unlikely to bias language change strongly enough to drive out tonal languages within 5,800 years. Furthermore, if this were true, there ought to be evidence of widespread loss of tonal systems in the recent centuries. Given that there is no such evidence, nor is there a proposed mechanism of causality, it is argued that it is unlikely for Dediu & Ladd’s conclusion to be true.
Overall, it seems that there is little in the way of conclusive evidence for a singular gene group having such a drastic impact as to be able to affect the tonality of languages. However, as Dediu & Ladd add in their paper, they present their research as a proof of concept, meaning that they hope that others can build on their work and determine whether or not this is a fruitful path of research.
[Back to Table of Contents]
6.3 Microcephalin, ASPM and other correlations
Other research has suggested correlations between microcephalin, ASPM and other cognitive capabilities. A study by Woodley et al. (2014) has found that though microcephalin and ASPM alleles do not appear to be associated with IQ at the individual level, the frequencies of microcephalin appear to correlate strongly with IQ at the cross-country level.
There are a number of other correlations discovered, but they are primarily non-linguistic in nature and as such will not be mentioned in this article.
[Back to Table of Contents]
7. Conclusion
This chapter has dealt with language as a biological adaptation driven by natural selection processes. It has also evaluated theories and evidence in support. The two main theories dissected were the Theory of Mind, and the FOXP2 gene, and the funtions of the FOXP2 gene. This chapter also showed that Malle’s model is able to clarify many doubts in language evolution and also strengthened the theory of protolanguage. Theory of Mind’s compatibility with grammar provides a strong explanation for the complexity of today’s human language system. The study of FOXP2 will also help unlock a lot of doubts about the role of genes in the evolution of language. However, there is uncertainty of the function of FOXP2 itself, given that it is a transcription gene, and further research is needed. Therefore the Theory of Mind as explained by the Malle’s Model still winds up as the more compelling argument in this chapter.
[Back to Table of Contents]
References
Arden, J. B. (2010). Rewire Your Brain: Think Your Way to a Better Life [Digital Version]. Retrieved from https://books.google.com.sg/books?id=PlROeBZrVG4C&lpg=PP1&dq=AARP%20Rewire%20Your%20Brain%3A%20Think%20Your%20Way%20to%20a%20Better%20Life&pg=PP1#v=onepage&q=AARP%20Rewire%20Your%20Brain:%20Think%20Your%20Way%20to%20a%20Better%20Life&f=false
Bickerton, D. (2007). Language evolution: A Brief guide for linguists. Lingua, 117, 510-526.
Bruinsma, Y., Koegel, R. L., & Koegel, L. K. (2004). Joint attention and children with autism: A review of the literature. Mental Retardation and Developmental Disabilities, 10, 169–175.
Carroll, S. B. (2005). Evolution at Two Levels: On Genes and Form. PLoS Biology, 3(7), e245. doi:10.1371/journal.pbio.0030245
Carpenter, M., Nagell, K., & Tomasello, M. (1998). Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for
Research in Child Development, 63(4), Serial No. 255.
Currat, M., Excoffier, L., Maddison, W, Otto, S. P., Ray, N., Whitlock, M. C., and Yeaman, S. (2006). Comment on ‘Ongoing Adaptive Evolution of ASPM, a Brain Size Determinant in Homo sapiens’ and ‘Microcephalin, a Gene Regulating Brain Size, Continues to Evolve Adaptively in Humans’. Science 313: 172.
Dediu, D., & Ladd, D. R. (2007). Linguistic tone is related to the population frequency of the adaptive haplogroups of two brain size genes, ASPM and Microcephalin. Proceedings of the National Academy of Sciences, 104(26), 10944-10949. doi:10.1073/pnas.0610848104
de Villiers, J. G., & de Villiers, P. A. (2003). Language for thought: coming to understand false beliefs. In D. Gentner & S. Goldin-Meadow (Eds.), Language in Mind: Advances in the Study of Language and Thought (pp. 335-383). Massachusetts: MIT Press.
Diller, K. C., & Cann, R. L. (2011). Genetic influences on language evolution: An evaluation of the evidence. Oxford Handbooks Online. doi:10.1093/oxfordhb/9780199541119.013.0015
Evans, P. D., Gilbert, S. L., Mekel-Bobrov, N., Vallender, E. J., Anderson, J. R., Vaez-Azizi, L. M., . . . Lahn, B. T. (2005). Microcephalin, a Gene Regulating Brain Size, Continues to Evolve Adaptively in Humans. Science, 309(5741), 1717-1720. doi:10.1126/science.1113722
Fisher, S.E., Vargha-Khadem, F.,Watkins, K.E., Monaco, A.P. & Pembrey, M.E. (1998). Localisation of a gene implicated in a severe speech and language disorder. Nature-Genetics 18 (2): 168–170.
Gopnik M (1990). Feature‐blind grammar and dysphasia. Nature 1990, 344:715.
Haesler S., Rochefort C., Georgi B., Licznerski P., Osten P., & Scharff C.(2007) Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus area x. PLoS Biology 5(12) 2885-2897Johansson, S. (2013). The Talking Neanderthals: What Do Fossils, Genetics and Archeology say? Biolinguistics, 7, 35-74.
Hurst JA, Baraitser M, Auger E, Graham F, Norell S. An extended family with a dominantly inherited speech disorder (1990). Dev Med Child Neurol 1990, 32:352–355.
Kiester, E. (2001, January). Accents Are Forever. Retrieved from https://www.smithsonianmag.com/science-nature/accents-are-forever-35886605/
Lai C.S. Fisher S.E., Hurst J.A., Vargha-Khadem F. & Monaco A.P. (2001) A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413(6855): 519–23
Malle, B. F. (2004). The relation between language and theory of mind in development and evolution. In T. Givon & B. F. Malle (Eds.), The evolution of language out of pre-language (pp. 265-284). Amsterdam: Benjamins.
Marcus, G. F., & Fisher, S. E. (2003). FOXP2 in focus: what can genes tell us about speech and language? Trends in Cognitive Sciences, 7(6), 257-262. doi:10.1016/s1364-6613(03)00104-9
Merrian Webster. (n.d.). Medical Definition of Upregulation. Retrieved from https://www.merriam-webster.com/medical/upregulation
Miller, C. A. (2006). Development relationships between language and theory of mind. American Journal of Speech-Language Pathology, 15, 142-154.
Mori, C., & Wada, K. (2015). Songbird: a unique animal model for studying the molecular basis of disorders of vocal development and communication. Experimental Animals, 64(3), 221-230. doi:10.1538/expanim.15-0008
Pinker, S. (2003). Language as an adaptation to the cognitive niche. In S. Kirby & M. Christiansen (Eds.), Language evolution: States of the Art, (pp. 16-37). New York: Oxford University Press.
Pinker, S., & Bloom, P. (1990). Natural Language and Natural Selection. Behavioural and Brain Sciences, 13(4), 707-784.
Penn, D. C., Holyoak, K. J., & Povinelli, D. J. (2008). Darwin’s Mistake: Explaining the discontinuity between human and nonhuman minds. Behavioural and Brain Sciences, 31, 109-178.
University of Leicester. (n.d.). Gene Expression and Regulation. Retrieved from https://www2.le.ac.uk/projects/vgec/highereducation/topics/geneexpression-regulation
Vargha‐Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R (1995). Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci U S A 1995, 92:930–933.
Webb, D. M., & Zhang, J. (2004). FoxP2 in Song-Learning Birds and Vocal-Learning Mammals. Journal of Heredity, 96(3), 212-216. doi:10.1093/jhered/esi025
White, S. A. (2010). Genes and vocal learning. Brain and Language, 115(1), 21-28. doi:10.1016/j.bandl.2009.10.002
Wolfgang, E., Przeworski, M., Fisher, S., Lai, C., Wiebe, V., Takashi, K., . . . Pääbo, S. (2002). Molecular evolution of FOXP2, a gene involved in speech and language. Nature, 869-872.
Woodley, M. A., Rindermann, H., Bell, E., Stratford, J., & Piffer, D. (2014). The relationship between Microcephalin, ASPM and intelligence: A reconsideration. Intelligence, 44, 51-63. doi:10.1016/j.intell.2014.02.011
Yu, F., Hill, R. S., Schaffner, S. F.,Sabeti, P. C.,Wang, E. T.,Mignault, A. A., Ferland, R. J., Moyzis, R. K., Walsh, C. A., and Reich, D. (2007). Comment on ‘Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens’. Science 316: 370.
[Back to Table of Contents]

