Types of fossil fuels
1. Petroleum
Petroleum in its raw form is called crude oil. Both petroleum and crude oil have a complex structure of hydrocarbons. The varying hydrocarbon structures account for the different characteristics and properties that petroleum and crude oil has. [1]
There are 4 main hydrocarbons that can be found in crude oil :
i.paraffins a.k.a alkanes(15-60%) [2]
ii.naphthenes a.ka cycloalkanes(30-60%)
iii.aromatics(3-30%)
iv.asphaltics(3-10%)[3]
2. Oil and natural gas
Both oil and natural gases are generally formed when marine organisms die and they settle to the bottom of the seabed. Anaerobic environment prevents the decomposition of the bodies and they get buried by sea sediments. After prolong periods of time under great pressure and heat from the core of the Earth, the bodies’ molecules start to break down into kerogen which will further evolve into hydrocarbons. The only difference in the formation of oil and natural gas is that natural gas is formed much nearer to the core of the Earth(usually depths of more than 10km below seabed as compared to 1 – 6 km for oil).[4]

Figure 1: Shows the rough depths of where oil and natural gases can be found
Fun Fact 3: The energy stored in fossil fuels comes from the sun.[5]
The following are the physical properties of crude oil:
- Freezing and boiling points. When heating at 100 kPa a frozen crude-oil sample (from below 210 K), solid-liquid equilibrium may exist in the range 210 K to 280 K, and liquid-vapour above 280 K; vapours start to decompose at about 900 K.
- Composition. Each crude-oil field has a different composition, that can be established by a combination of gas-chromatography, fluorescence-spectroscopy and infrared-spectroscopy techniques, and that may be used, for instance, in forensic analysis of oil spills at sea (even after refining, crude-oil derivatives may be associated to their source field). Saturated hydrocarbons content is around 60%wt, aromatics 30%wt, resins 5%wt. Sulfur content is 0.5 to 2%wt. Heavy metals <100 ppm. Crude-oil vapours are mainly short-chain hydrocarbons (only about 10% in volume have more than 4 carbons).
Natural gas is a flammable gaseous mixture, composed mainly of methane: CH4, C2H6, C3H8, and minor concentrations of H2O, CO, CO2, N2 and He. It is found on many underground cavities, either as free deposits (e.g. Indonesia, Algeria, New Zealand) or linked to petroleum fields (e.g. Saudi Arabia, Nigeria, Alaska). [6]
3. Coal
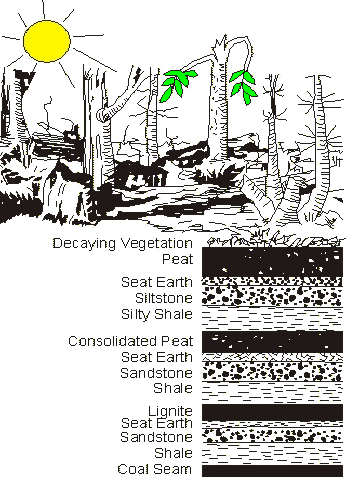
Figure 2: Shows the formation of coal and the different stages
Coal originated from plant debris such as ferns, trees, bark and leaves, some of which accumulated and settled in swamps.
This unconsolidated accumulation of plant remains is called peat. Peat is being formed today in marshes and bogs.
Layers of peat are covered by sediments receiving heat and pressure from the subsidence of the swamps, which undergoes a process called coalification to form coal.
The process occurs in several stages. The conditions of the process, the swamps and bogs affect the formation of the coal.
Coal formed from peat initially has a high moisture content and a relatively low heating value. [7]
Combustion
The combustion of all fossil fuels follows a general reaction:
The reaction of fuel (any hydrocarbon source) with oxygen yields carbon dioxide and water and energy as its products.
A simple combustion reaction is given for methane. When this reaction takes place, the result is carbon dioxide (CO2), water (H2O), and a great deal of energy. The following reaction represents the combustion of methane:
CH4[g] + 2 O2[g] -> CO2[g] + 2 H2O[g] + energy
One molecule of methane, combines with two oxygen molecules, to form a carbon dioxide molecule, and two water molecules are given off as steam or water vapor during the reaction.[8]
Figure 3: Shows the combustion reaction of methane
Cracking
Cracking is process of breaking up large hydrocarbon molecules into smaller molecules. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst.
The source of the large hydrocarbon molecules is often the naphtha fraction or the gas oil fraction from the fractional distillation of crude oil (petroleum). These fractions are obtained from the distillation process as liquids, but are re-vaporised before cracking.
Cracking can occur in many different ways. The hydrocarbon molecules are broken up in a random way to produce mixtures of smaller hydrocarbons, some of which have carbon-carbon double bonds.[9]
One possible reaction involving the hydrocarbon C15H32 might be:
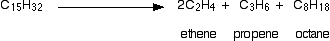
Figure 4: Showing the cracking of C15H32
Or, showing more clearly what happens to the various atoms and bonds:
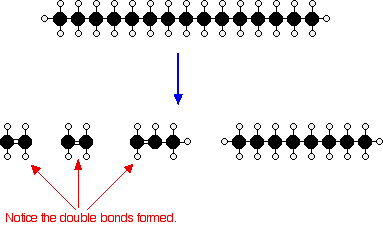
Figure 5: Showing cracking of C15H32 in atomic and bond level
Distillation
Distillation is made possible because of the different boiling points of the alkanes. The longer the carbon chains, the higher the boiling point due to higher intermolecular forces between the molecules. Due to this difference in boiling point, the crude oil is able to be fractionally separated with the products at the top being the lowest boiling point and the products at the bottom having the highest boiling point.

Figure 6: Shows the standard distillation process of crude oil
References
1.http://chemistry.about.com/od/geochemistry/a/Chemical-Composition-Of-Petroleum.htm
2.http://global.britannica.com/EBchecked/topic/442584/paraffin-hydrocarbon
3.http://www.eoearth.org/view/article/153618/
4.http://www.learner.org/courses/envsci/unit/text.php?unit=10&secNum=4
5. http://www.softschools.com/facts/energy/fossil_fuel_facts/407/
6.http://www.coaleducation.org/Ky_Coal_Facts/coal_resources/coal_origin.htm
7.http://webserver.dmt.upm.es/~isidoro/bk3/c15/Fuel%20properties.pdf
8.http://www.elmhurst.edu/~chm/vchembook/511natgascombust.html
9.http://www.chemguide.co.uk/organicprops/alkanes/cracking.html
Figures
1.http://www.learner.org/courses/envsci/visual/visual.php?shortname=hydrocarbon_system
2.http://www.coaleducation.org/Ky_Coal_Facts/coal_resources/coal_origin.htm
3.http://www.elmhurst.edu/~chm/vchembook/511natgascombust.
4.http://www.chemguide.co.uk/organicprops/alkanes/cracking.html
5.http://www.chemguide.co.uk/organicprops/alkanes/cracking.html
6.http://www.glogster.com/media/4/13/58/68/13586885.jpg
Header Image:
http://st.depositphotos.com/1389325/1280/v/950/depositphotos_12809674-chemistry-background—molecule-models-and-formulas.jpg

