Like any other organism, aquatic life forms need a stable pH in their environment. In other words, aquatic organisms need the pH of their water body to be within a certain range for optimal growth and survival. Although each organism has an ideal pH, most aquatic organisms prefer pH of approximately 6.5 – 8.0. Outside of this range, organisms become physiologically stressed. Reproduction can be impacted by out-of-range pH, and organisms may even die if the pH gets too far from their optimal range. In addition to directly affecting the physiology of aquatic organisms, additional aspects of lake dynamics are influenced by pH. Low pH can cause the release of toxic elements and compounds from sediments into the water where they may be taken up by aquatic animals or plants. Changes in pH also influence the availability of plant nutrients, such as phosphate, ammonia, iron and trace metals, in the water.
However, there are many factors which may significantly alter this parameter leading to unsuitable conditions for fish and sea plants to thrive in water. One of the major problems is pollution in the form of acid rain. This acidity is derived from sulphur dioxide (SO2) emissions which are converted to sulphuric acid (H2SO4) in the clouds and dissolved in rain water.
SO2 + O3 (from the sun) -> H2SO4 -> H+ + SO42-
When a body of water is affected by acid rain, its natural buffering systems get into operation. This includes, for example, the reaction between carbonate (CO32- and HCO3–) and hydrogen (H+) to neutralise its effects.
CO32- + H+ -> HCO3– + H+ -> H2CO3
—————————————————————————————————–
This buffering capacity of a water body is known as its alkalinity. It measures the ability of water bodies to neutralize acids and bases thereby maintaining a fairly stable pH. Water that is a good buffer contains compounds, such as bicarbonates, carbonates, and hydroxides, which combine with H+ ions from the water, thereby raising the pH (more basic) of the water. Without this buffering capacity, any acid added to a lake would immediately change its pH.
Alkalinity comes from rocks and soils, salts, certain plant activities, and certain industrial wastewater discharges (detergents and soap-based products are alkaline). If an area’s geology contains large quantities of calcium carbonate (CaCO3, limestone), water bodies tend to be more alkaline. On the other hand, granite bedrock is deficient in alkaline materials to buffer acidic inputs. Addition of lime as a soil amendment to decrease acidity in home lawns can also runoff into surface waters and increase alkalinity.
—————————————————————————————————–
However, this reaction has a limited influence on the pH, and if it falls outside its buffer capacity, extreme pH (either highly acidic or highly alkaline) can cause irreversible damage to aquatic life, particularly affecting gills and fins in fish.
The pH in soil is also critical, as plants grow better under specific pH conditions. Also caused by acid rain, acidification of the soil is becoming a serious problem, as natural buffer systems may not be able to cope with such drastic changes. This way, not only vital nutrients are dissolved and washed away, but also the micro-organisms responsible for nitrogen fixation and breakdown of organic matter are affected, eventually disturbing the entire ecosystem.
Type of Soil Buffers:
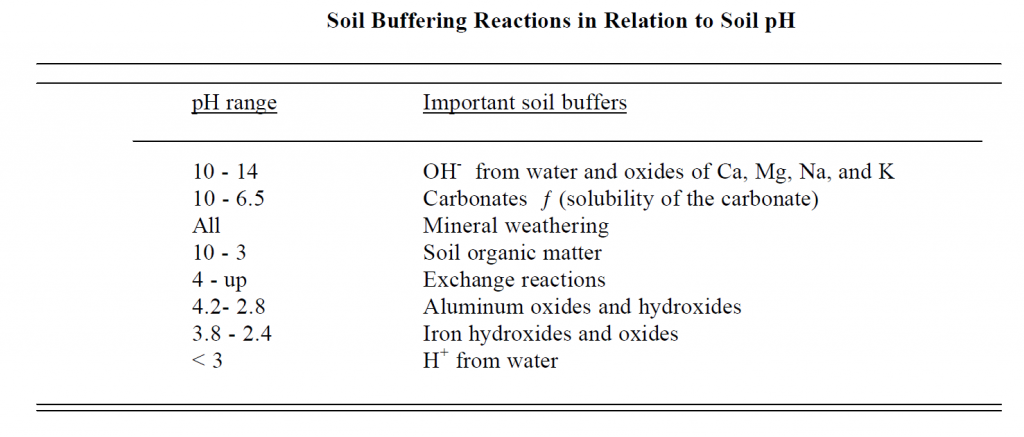
For increasing pH:
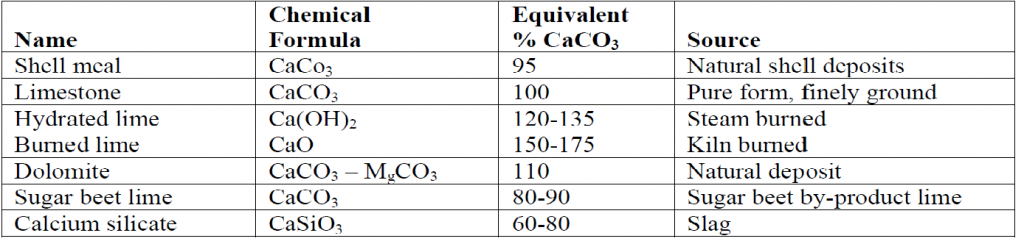
The most common amendment to increase soil pH is lime (CaCO3 or MgCO3), usually in the form of finely ground agricultural lime. Calcitic limestone (CaCO3 ) provides a good source of Calcium (Ca) and helps neutralize soil acidity while dolomitic limestone functions similarly but also adds Magnesium (Mg).
Some common liming materials are listed in the table below.
The amount of lime needed to change pH is determined by the mesh size of the lime (how finely it is ground)and the buffering capacity of the soil. A high mesh size (60–100) indicates a finely ground lime, that will react quickly with soil acidity. Buffering capacity of soils is a function of a soil’s cation exchange capacity, which is in turn determined by the clay content of the soil, the type of clay and the amount of organic matter present. Soils with high clay content, particularly shrink-swell clay, will have a higher buffering capacity than soils with little clay. Soils with high organic matter will also have a higher buffering capacity than those with low organic matter. Soils with high buffering capacity require a greater amount of lime to be added than a soil with a lower buffering capacity for the same incremental change in pH.
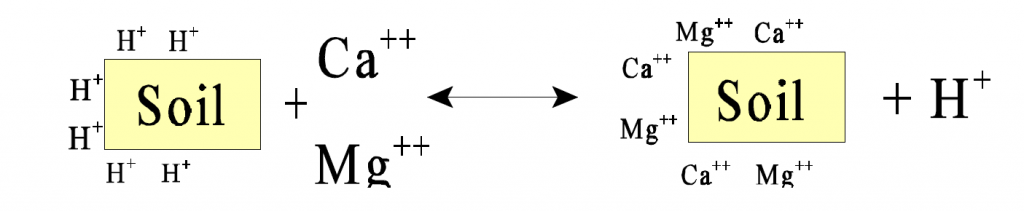
The chemistry to liming is quite simple. Hydrogen ions (H+) are attracted to soil and organic material which have a negative charge. When lime is applied, these hydrogen ions are exchanged for calcium or magnesium (Ca2+ or Mg2+) ions which have a greater positive charge. This helps to neutralize the acidity of the soil. The free hydrogen ions are taken out of solution. This also helps to increase the pH.
This reaction demonstrates the process of liming:
Other amendments that can be used to increase the pH of soil include wood ash, industrial CaO (burnt lime), and oyster shells. White firewood ash includes metal salts, which are important for processes requiring ions, such as Na+ (sodium), K+ (potassium), Ca2+ (calcium), which may or may not be good for the select flora, but will indeed decrease the acidic quality of soil.
For decreasing pH:
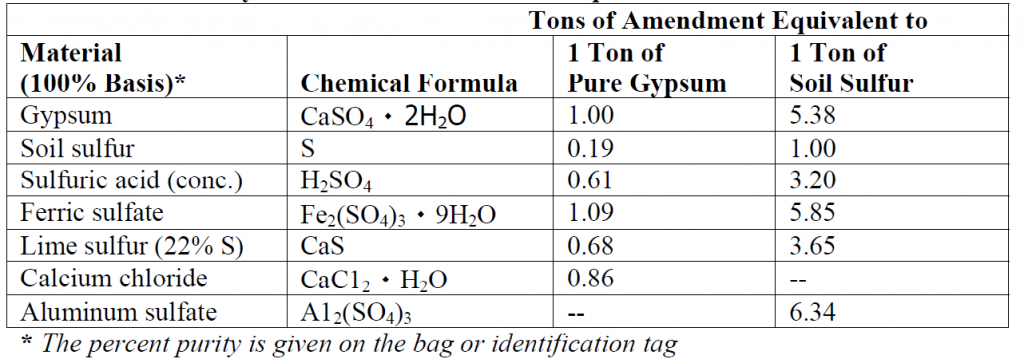
Some soils are alkaline and have a pH above 6.5. Some fertilizers (ammonium sulphate, urea, and ammonium nitrate) create an acid reaction in the soil, so they aid in lowering or maintaining a specific pH. Certain acidifying organic materials, such as pine needles or peat moss can lower soil pH gradually over many years. In nature, this takes thousands of years. For more rapid results in lowering pH, sulphur is used. Sulphuric acid forms when sulphur is added to the soil, the smaller the particles of sulphur, the faster the reaction. Lowering the pH is a slow process and will take 1-2 years to see a reaction.
The table below shows some commonly used materials to decrease the pH of soil:
Impacts of soil pH:
As mentioned earlier, without buffers to help to maintain the pH of soil, the consequences are very adverse.
Some areas affected by soil pH includes:
- Plant Response :
A soil pH(CaCl2) of 5.2 to 8.0 provides optimum conditions for most agricultural plants, as shown in the table below.
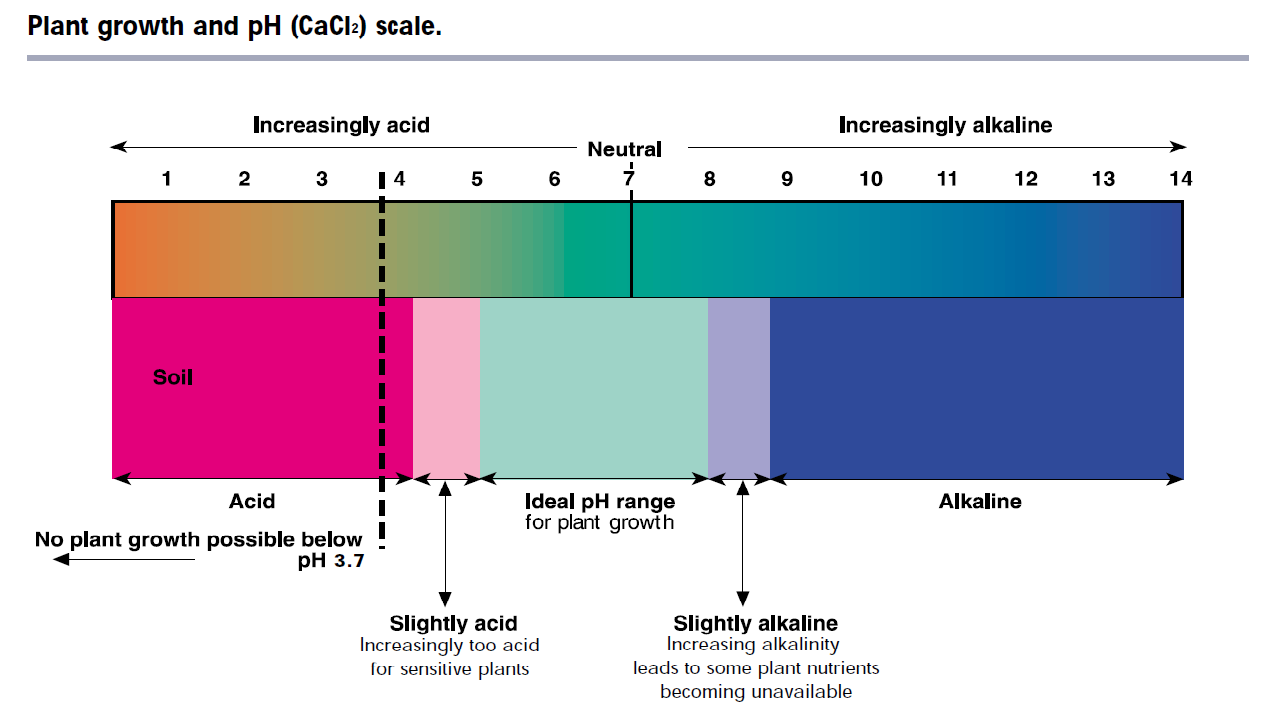
All plants are affected by the extremes of pH but there is wide variation in their tolerance of acidity and alkalinity. Some plants grow well over a wide pH range, whilst others are very sensitive to small variations in acidity or alkalinity.
The figure below provides a guide to the preferred pH(CaCl2) for some common crops and pastures.
Microbial activity in the soil is also affected by soil pH with most activity occurring in soils of pH 5.0 to 7.0. Where the extremities of acidity or alkalinity occur, various species of earthworms and nitrifying bacteria disappear. Legume root colonising bacteria (Rhizobia) vary in their sensitivity to soil pH and have preferred ranges in which they are effective. In some crops and pastures (e.g. faba beans and lucerne) the Rhizobia specific to these plants are more sensitive than the plant itself.
- Availability of Soil nutrients
Soil pH affects the availability of nutrients and how the nutrients react with each other, as illustrated in the figure below.
At a low pH, beneficial elements such as molybdenum (Mo), phosphorus (P), magnesium(Mg) and calcium (Ca) become less available to plants. Other elements such as aluminium (Al), iron (Fe) and manganese (Mn) may become more available and Al and Mn may reach levels that are toxic to plants. Specifically, a small drop in pH can result in a large increase in soluble aluminium. In this form, aluminium retards root growth, restricting access to water and nutrients. Poor crop and pasture growth, yield reduction and smaller grain size occur as a result of inadequate water and nutrition. The changes in the availability of nutrients cause the majority of effects on plant growth attributed to acid soils. Sensitive crops such as barley and Lucerne can be affected by small amounts of exchangeable aluminium.
In contrast, when the pH(CaCl2) is greater than 7.5, calcium can tie up phosphorus, making it less available to plants. Additionally, alkaline soils cause zinc and cobalt deficiencies that lead to stunted plants, poor growth and reduced yields in some crops and pastures.
Other implications to society:
(Click on each of these to dwell into the specific implication of buffers to society)
1. Buffers and Industrial Applications
2. Buffers in Human Body
References:
- http://environmental-realm.blogspot.sg/2012/04/importance-of-buffers-in-physiological.html
- http://www.thechemicalblog.co.uk/buffers-in-our-daily-life/
- http://www.uri.edu/ce/wq/ww/Publications/pH&alkalinity.pdf
- http://lawr.ucdavis.edu/classes/ssc102/Section8.pdf
- http://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/167187/soil-ph.pdf
Pictures:
- http://www.thechemicalblog.co.uk/buffers-in-our-daily-life/
- http://upload.wikimedia.org/wikipedia/commons/thumb/c/c0/BiyaRiver.JPG/640px-BiyaRiver.JPG
- http://vric.ucdavis.edu/pdf/Soil/ChangingpHinSoil.pdf
- http://www.edu.pe.ca/agriculture/soil.pdf
- http://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/167187/soil-ph.pdf
- http://lawr.ucdavis.edu/classes/ssc102/Section8.pdf