Apart from the characteristics, we can tell whether a solution is acidic or alkaline by looking at its pH value. What does pH stands for?
pH is the negative logarithm of the activity or the concentration of the hydrogen ion in an aqueous solution (with the mathematical expression of -log[H+]). It is often used as a measurement of concentration hydrogen ions in the solution.
The below chart gives us some examples of different pH values.

The pH value ranges from 0 to 14. The pH range tells us the acidity of the solution:
1) Acidic – pH < 7
2) Neutral – pH = 7
3) Alkaline/Basic – pH > 7
If you recall the chemistry experiments you have done back in your high school days, you will probably know that we use some visual aid to determine to range of pH values. The visual aid are the pH indicators which change colours when the pH of the solution changes at a certain range. There are often used to determine the completion of the neutralisation between certain acids and bases, e.g. titration. Let’s look into the different pH indicators and their transition pH ranges.
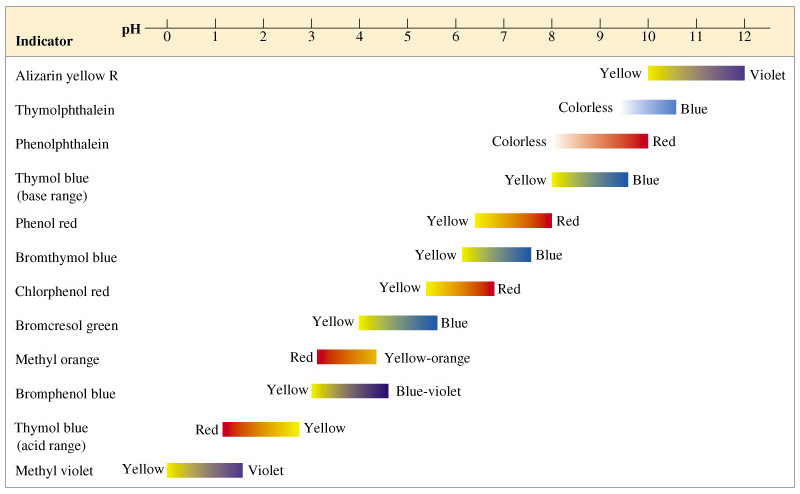
pH indicators
References:
Content:
http://www.elmhurst.edu/~chm/vchembook/184ph.html
Pictures:
https://aegeanpoolsretail.files.wordpress.com/2012/09/phscalecomparison.jpg
http://mtpleasant.glk12.org/mod/resource/view.php?id=7515
