Introduction
Fuels made from oil mixtures containing large hydrocarbon molecules are inefficient. They do not flow easily and are difficult to ignite. Crude oil often contains too many large hydrocarbon molecules and are not enough small hydrocarbon molecules to meet demand.
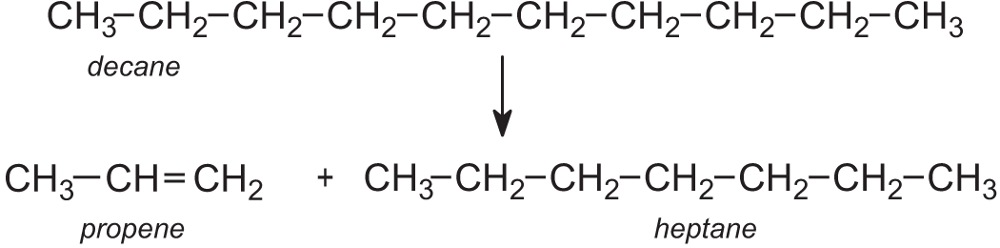
Fractions that are produced by the distillation of crude oil can go through a process called cracking. This chemical reaction produces smaller hydrocarbons, including alkanes and alkenes.
There are a several types of cracking – Thermal Cracking, Catalytic Cracking, Hydrocracking etc.
Explanation


References
Cleveland, C. (2013). Cracking. Retrieved March 22 from, http://www.eoearth.org/view/article/151525/
Freudenrich,C. (2001). How Oil Refining Works. Retrieved March 22 from, http://science.howstuffworks.com/environmental/energy/oil-refining5.htm
Polymers and ethanol from oil. (n.d.). Retrieved March 22, from http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev2.shtml






