Just earlier this year (2018 at point of writing), an exciting discovery has been made! A new form of steroisomerism has been discovered. This form of isomerism is known as Akamptisomerism and has only been found in porphyrin–boron complexes [1]. A huge reason could be due to the fact that this form of isomerism is only recently discovered and more work might be done in the future to discover more ways this form of isomerism can manifest in.
It is interesting to note that this form of isomerism is actually discovered by chance. The chemists who initially discovered this was actually working on porphyrin–diboron complexes for electronic devices [2]. After synthesis of these isomers, they realised that they were unable to name these compounds using standard nomenclature, and this is how Akamptisomerism was born.
Named after the Greek word for inflexible, these Isomers are based on hindered bond angle inversion. In the compounds studied, the bulky porphyrin rings do not allow for free rotation about the boron-oxygen bonds.
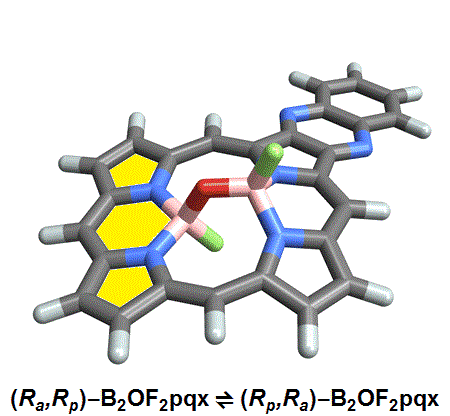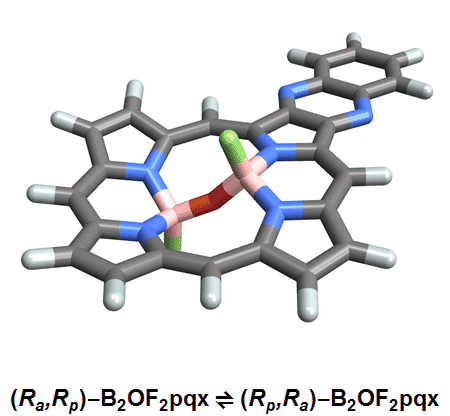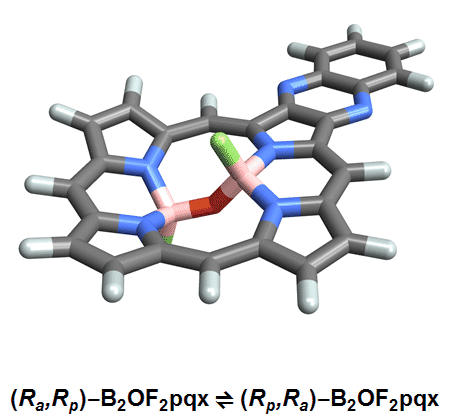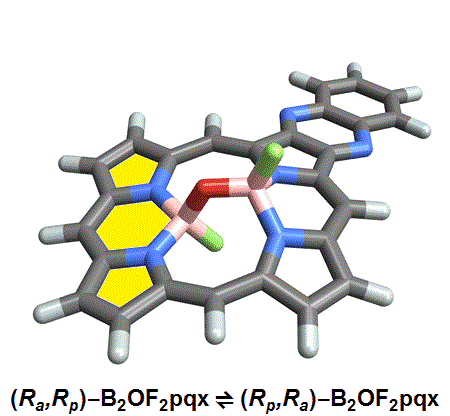
Of the eight different isomers that they predict to exist, the chemists have managed to synthesize four of them. Density funcctional calculations have been done on the other four compounds and have shown that they are less stable, indicating that it might be very difficult, if not impossible, to synthesize them [1].
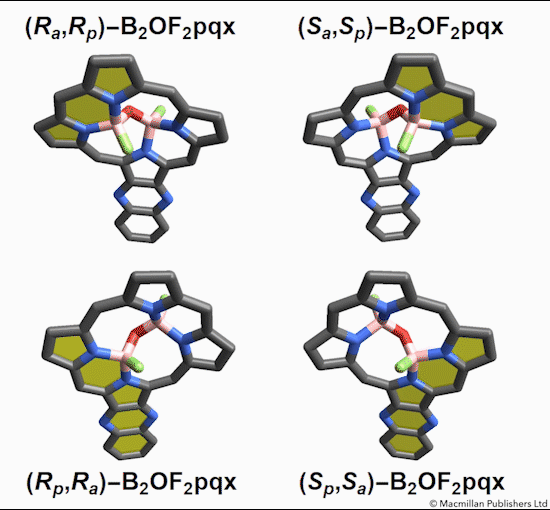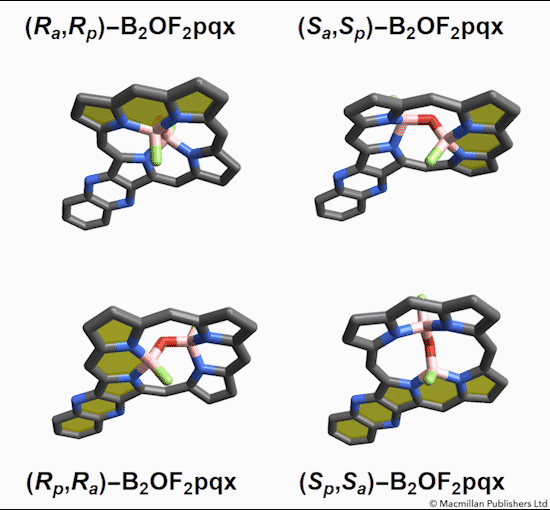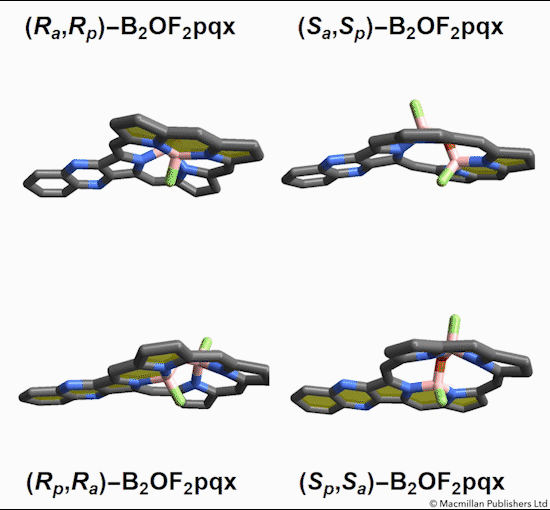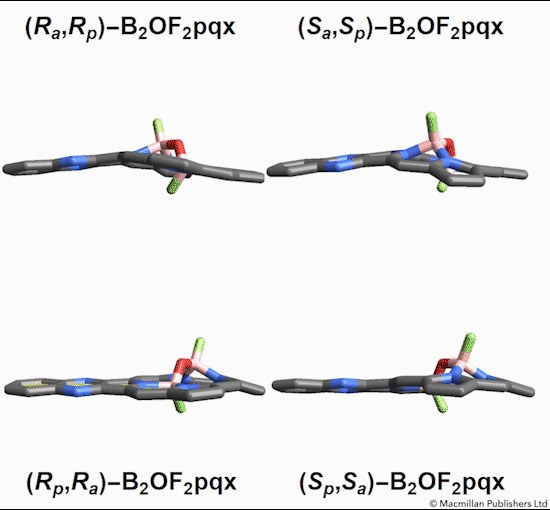
This form of isomerism is special in the sense that the application of heat can convert one form of diastereomer to another. Heating to around 50°C will turns one diastereomer into the other through bond angle inversion. The oxygen atom pushes through the porphyrin plane through a transition state where the B-O-B bond is linear[3]. Usually, molecules with bent bonds will interconvert through double torsion (twisting), which occurs too quickly for individual enantiomers to be isolated. However, the akamptisomers’ porphyrin ring makes torsion impossible, and thus the bond angle inversion is slow enough to make it possible to isolate isomers [1].
Although this form of isomerism is in its infancy, there are many projected applications for them. The ability for the enantiomers to interconvert from one form to another allows for it to see potential in medicinal chemistry or for use as molecular switches. Further work will definitely need to be done to determine the feasibility of such methods and the degree of control we have over the interconversion of the enantiomers, but it is definitely extremely exciting to see them integrated into our daily lives.
Seeing new forms of isomerism discovered even today is definitely exciting and inspiring. Although it is claimed that this form of isomerism will be the last form of isomerism to be discovered, I wouldn’t be surprised if newer forms of isomerism still continues to be discovered in the future.