Now, let us delve deeper into the concept of enantiomers. Enantiomers refer to isomers that are non-superimposable mirror images of one another. Each pair of enantiomers have almost identical chemical and physical properties, but differ in the way they rotate plane-polarised light and may behave differently in chiral environments.
Take our hands for example, they appear to be the same, each made up of a thumb, and four fingers. But putting one hand over the other shows they are not quite the same. Our left and right hands are mirror images of each other, but are non-superimposable over the other [1], as illustrated in the diagram below.
Likewise, enantiomers consist of the same chemical formula, but their different spatial arrangement makes them non-superimposable over the other. Another way to look at this is to attempt to rotate one isomer and see if you can end up with the same 3D structure of the other. It is observed that the 2 remaining groups have swapped positions, and this proves that they are non-superimposable [2].
A molecule with a non-superimposable mirror image is typically referred to as a chiral molecule. A key feature that is often the cause of chirality in molecules is the presence of an asymmetric carbon atom. An asymmetric carbon atom is one that attaches to four different types of atoms or groups of atoms. On the other hand, achiral molecules typically have a plane of symmetry and hence, have superimposable mirror images. An chiral molecule that also has a chiral center is known as a meso compound.
Referring to Figure 2 above, Bromochlorofluoromethane is a chiral molecule and rotation of its mirror image does not generate the original structure [2]. If one were to flip over the left molecule over to the right, the atomic spatial arrangement will not be equal. This is equivalent to the left hand – right hand relationship. To superimpose the mirror images, bonds must be broken and reformed. Hence, the 2 molecules are enantiomers of each other. In contrast, dichlorofluoromethane and its mirror image can be rotated, hence they are superimposable. Therefore, it is an achiral molecule.
Enantiomers have a chiral centre that is surrounded by 4 different atoms or groups. A simple example of chiral centers would involve a chiral carbon, as illustrated with an asterisk on the Bromochlorofluromethane molecule above. However, other chiral centres include nitrogen and phosphorus [3].
Chirality at Nitrogen
Single-bonded nitrogen is pyramidal in shape, with the non-bonding electron pair pointing to the unoccupied corner of a tetrahedral region. If the nitrogen is bonded to three different groups, its configuration is chiral. The non-identical mirror-image configurations are illustrated in the diagram above. If these configurations were stable, there would be additional stereoisomers. However, pyramidal nitrogen is normally not configurationally stable. It rapidly inverts its configuration (as shown by the equilibrium arrows) at room temperature, by passing through a planar, sp2-hybridized transition state, leading to a mixture of interconverting R and S configurations [3]. This is known as amine inversion. Thus, if the nitrogen atom were the only chiral center in the molecule, there would not be isolable stereoisomers as the amine inversion occurs at a very fast rate at room temperature [4].
Chirality at Phosphorus
Due to the tetrahedral shape about the phosphorus center of phosphate ion and organic phosphate esters, it is potentially a stereocenter too [3]. When phosphorus has 4 different groups, it is a chirality center, and thus, the compounds can exists as enantiomers.
R and S Configurations
You may have realised that we mentioned R and S configurations earlier on, but what exactly is that? Stereocenters of chiral molecules are labeled as R or S. The groups around the chiral centre are assigned priority based on their atomic number, with the lowest, such as a hydrogen atom, being priority #4. In R isomers, #1 to #3 are arranged in a clockwise manner, while in S isomers, they are arranged in an anticlockwise manner with the #4 priority group projected into the plane away from you. R/S configurations can only be derived from observation of the 3D configuration of the molecule [2].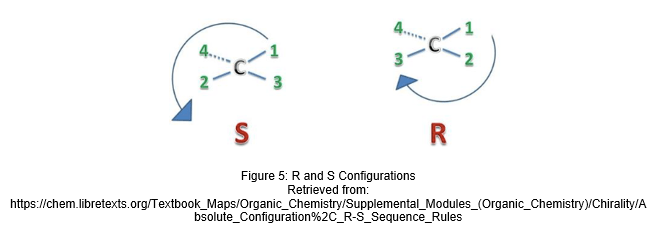
Dextrorotatory or Levorotatory?
As mentioned earlier, enantiomers differ in the direction in which they rotate plane-polarised light. An enantiomer that rotates plane-polarised light in the clockwise direction is called dextrorotatory (+) while the enantiomer that rotates the light in the counterclockwise direction is called levorotatory (-) [5]. When both isomers are present in equal amounts, the mixture is called a racemic mixture. Racemic mixtures are unable to rotate plane polarised light because although the two enantiomers rotate plane-polarised light in opposite directions, the rotations cancel as they are present in equal amounts. Whether an enantiomer is dextrorotatory or levorotatory can only be determined by observing the rotation of plane polarised light [5].
Rotation of Plane Polarised Light
As shown in the diagram above, the unpolarised light passes through a filter (polarizer) such that only waves that oscillate in a specific direction can pass through [5]. When these waves interact with an optically active material, like an enantiomer, they are rotated either counterclockwise or clockwise, depending on the enantiomer. In the case of the diagram above, the light is rotated clockwise, therefore the substance is a dextrorotatory enantiomer.
But why are enantiomers able to rotate plane-polarised light? What happens is this: when light passes through matter, for example, a solution containing chiral molecules, the light interacts with each molecule’s electron cloud, and these very interactions result in the rotation of the plane of oscillation for a ray of light [5]. The direction and magnitude of rotation depends on the nature of the electron cloud [5].
References
- Calmes, J., J. Roe, and A. Wang. Optical Isomerism. Available from: https://brilliant.org/wiki/optical-isomerism/.
- Chong, D. and J. Mooney. Chirality and Stereoisomers. 2016; Available from: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/ Supplemental_Modules_(Organic_Chemistry)/ Chirality/Chirality_and_Stereoisomers.
- Kennepohl, D., W. Reusch, S. Farmer, and T. Soderberg. Chirality at Nitrogen, Phosphorus, and Sulfur. 2018; Available from: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/ Map%3A_Organic_Chemistry_(McMurry)/ Chapter_05%3A_Stereochemistry_at_Tetrahedral_Centers/ 5.10%3A_Chirality_at_Nitrogen%2C_ Phosphorus%2C_and_Sulfur.
- Nitrogen and Phosphorus Chirality Centers. 2016; Available from: http://www.kshitij-iitjee.com/Nitrogen-and-Phosphorus-Chirality-Centers/.
- Natt, N.J. and A. Zhu. Optical Activity. 2018; Available from: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/ Supplemental_Modules_(Organic_Chemistry)/ Chirality/Optical_Activity.



