Welcome to another blog post! We hope that you have been having a great learning experience so far. In the past few blog entries, we have introduced the many kinds of stereoisomers. In today’s blog entry, we will not be introducing a new kind of stereoisomer but instead see how stereochemistry is involved in determining the products of various reactions!
To start off, some reactions can be classified as stereospecific, stereoselective or both.
What is a Stereoselective reaction?
In a stereoselective reaction, one stereoisomer product is formed selectively over other stereoisomers [1]. Depending on the relative amount of each stereoisomer formed as a product, the reaction can range from being completely stereospecific to being just moderately stereospecific.
An example of a stereoselective reaction is the reduction of hex-3-yne to hex-3-ene [1]. When using the H2/Lindlar catalyst, the main product formed is the Z-isomer of the alkene [1]. However, when Na/NH3 is used for the reduction, the main product becomes the E-isomer of the alkene instead [1]. This is due to the reagents being specific in how they add the hydrogen atoms to the C≡C bond leading to stereoselectivity.
So, what is a Stereospecific reaction?
For stereospecific reactions, the different stereoisomers of the reactants will react differently. To put it in another way, the stereochemistry of the reactant will determine the product of the reaction. One stereoisomer of the reactant will produce a specific stereoisomer of the product. When another stereoisomer of the reactant is used, it produces a reactant that is another stereoisomer of the product. As such, stereospecificity is a description of the mechanism of the reaction.
A classic example of a stereospecific reaction is the SN2 mechanism which refers to a bimolecular nucleophilic substitution [2]. In the case of a SN2 reaction, there will always be an inversion of stereochemistry at the chiral centre.
To illustrate, we will look at how SN2 reaction takes place with halogenoalkanes (See Fig 1.). In a halogenoalkane, there would be a C-X bond, whereby X represents the halogen. During nucleophilic substitution, the nucleophile would attack the carbon in the C-X bond since there is a partial positive charge on the carbon atom due to the differences in electronegativity between carbon and the halogen. 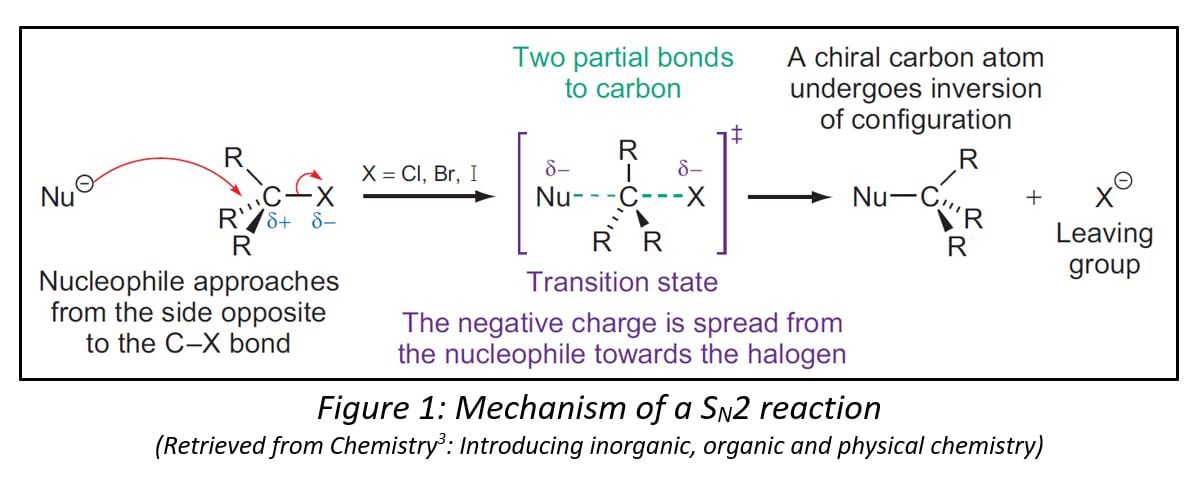
During the nucleophilic attack, the nucleophile approaches the carbon atom from the back of the halogen [3]. This will maximise the overlap of the orbital of the nucleophile with the empty σ* orbital of the C-X bond which is the most electron-accepting orbital [2]. Because of this attack on the opposite side of the halogen, there will be an inversion of configuration [3]. This highlights stereospecificity because if the S enantiomer is used at first, the R enantiomer will be produced and vice versa. 
All stereospecific reactions are also stereoselective. However, not all stereoselective reactions are stereospecific [2]. In order for a reaction to be stereospecific, there has to be stereoisomerism in the first place. In the case of the hex-3-yne in Figure 1, it does not have a stereoisomer so the reduction of hex-3-yne may be stereoselective, but it is not stereospecific. Another example of such a stereoselective but non-stereospecific will be described in the next section.
E2 Mechanism
An interesting mechanism to look at would be that of the E2 reaction. The E2 reaction is a biomolecular elimination reaction that takes place through a single-step mechanism. E2 reactions happen to halogenoalkanes in an anti-periplanar conformation, which results in the formations of alkenes [2].
The E2 reaction is stereoselective, but not stereospecific if there are 2 β hydrogens attached to the carbon in which H is eliminated from. In such a case, there are 2 possible anti-periplanar conformations that halogenoalkanes are able to adopt, which will produce either the E-isomer and Z-isomer of the alkene [2]. The E-isomer of the alkene is more stable than the Z-isomer due to less steric strain. As such, there is stereoselectivity as the E-isomer is formed in higher yield.
In the case where there is only one hydrogen on the β carbon, there would then be high stereospecificity of the reaction [4]. In such a case, the halogenoalkane can only adopt one anti-periplanar conformation. As such, only one isomer of the alkene will be obtained. This can be seen in the case of 1-bromo-1,2-diphenylpropane (Figure 3) where the each of the two diastereomers will result in only a specific E or Z alkene being produced.
Summary for E2 Mechanism
References
- Singh, K., P. Shakya, A. Kumar, S. Alok, M. Kamal, and S. Praksh. Stereochemistry and its role in drug design. 2014. International.
- Burrows, A., Holman, J., Parsons, A., Piling, G., and Price, G. (2017) Halogenoalkanes: Substitution and Elimination reactions, in Chemistry³: introducing inorganic, organic and physical chemistry 3rd ed. essay Oxford University Press Oxford.
- Stereochemistry SN2 Reactions. 2015; Available from: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/ Supplemental_Modules_(Organic_Chemistry)/Reactions/Substitution_Reactions/ SN2/Sterochemistry
- E2 Stereospecific. Available from: http://www.chemtube3d.com/EliminationE2stereospecific.html

