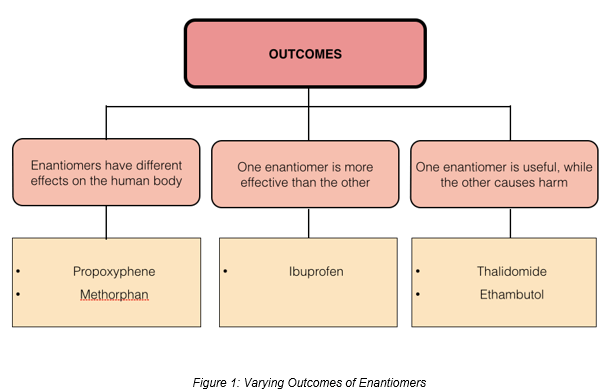
Stereochemistry is of particular importance in the pharmaceuticals industry. This is evident in the way enantiomerism in pharmaceutical products produces varying outcomes [1], as shown below.
01. Enantiomers that have different effects on the human body
Firstly, let us take a look at propoxyphene. The 2 enantiomers, dextropropoxyphene and levopropoxyphene have specific to different receptors, and hence elicit different responses in the human body [2]. Dextropropoxyphene, also known as Darvon, is marketed as an analgesic, which serves the purpose of pain relief. On the other hand, levopropoxyphene which was once marketed as Novrad has a different pharmocological effect. It serves as an antitussive which helps to relief coughs.
Similarly, the enantiomers of methorphan show different pharmacological and toxicological effects. Dextromethorphan is an antitussive commonly utilized in numerous over-the counter cough and cold medicines as it affects the signals in the brain that trigger the cough reflex. On the other hand, lextromethorphan is an opioid analgesic of the morphinan family that has never been marketed. It acts on opioid receptors to produce morphine-like effects.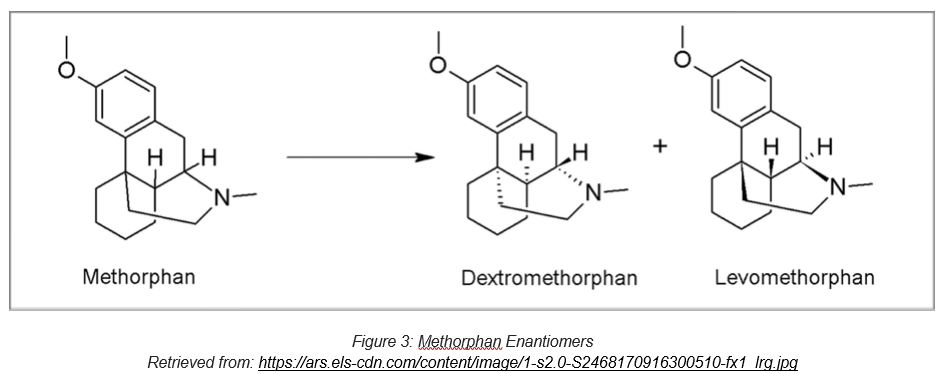
02. Cases where one enantiomer is more effective than the other
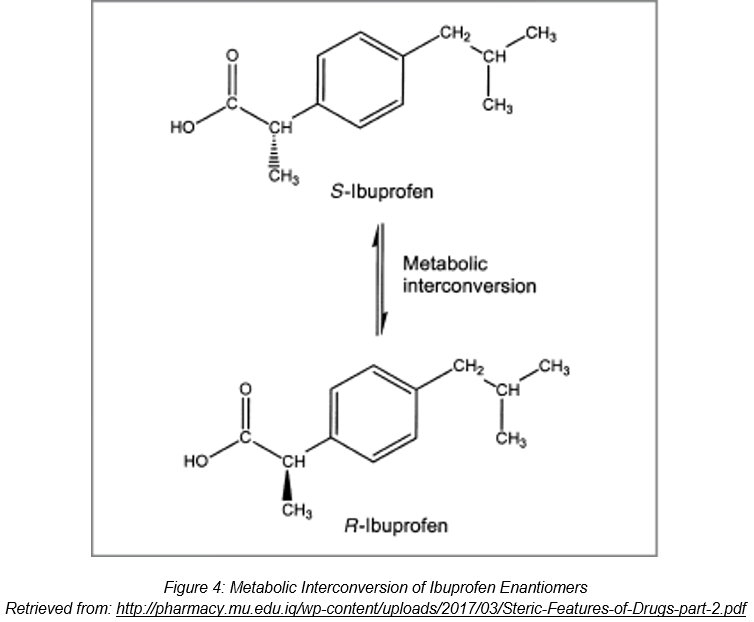
Let us now take a look at the varied effectiveness of the ibuprofen enantiomers. Ibuprofen is a non–steroidal anti-inflammatory drug that is used to treat conditions such as headaches and muscle pains. Ibuprofen exists as 2 enantiomers— the R(-) ibuprofen and the S(+) ibuprofen. Of the two enantiomers, S-ibuprofen is a more potent inhibitor of the cyclooxygenase enzyme than R-ibuprofen [2], and hence is more effective in eliciting a response.
Ibuprofen is sold as a racemic mixture of R(-) ibuprofen and S(+) ibuprofen, as shown in Figure x below. In the body, about 40-60% of R(-) ibuprofen is metabolically converted into S(+) ibuprofen in the intestinal tract after consumption, allowing ibuprofen to be more effective in reducing pains from inflammations [2].
However, this conversion of R(-) ibuprofen to S(+) ibuprofen could lead to a delayed onset of pharmacological activities, and there can also be difficulties in trying to obtain an optimal dosage of the drug [2]. Therefore, the availability of a single-enantiomer drug consisting of only the S(+) ibuprofen enantiomer would prevent this from happening, thus proving to be advantageous.
03. Cases where one enantiomer is useful, while the other is harmful
Did you know that there exists a medication that can treat you, but can also be lethal too? This infamous drug is known as thalidomide! Thalidomide was introduced in Germany in 1957 as a sedative and hypnotic, and was marketed over the counter largely as a drug for treating morning sickness in pregnant women, serving a useful purpose. However, in the following few years in which the drug was used by pregnant women, about 10,000 infants worldwide were born with phocomelia, or limb malformation. Only half of the infants survived, and some of those who did had other defects in addition to limb deficiencies [3].
How could a single drug result in 2 vastly different outcomes? Well, the reason behind the terrible effects is precisely because thalidomide exists as a racemic mixture of (R)- and (S)-enantiomers.
The (R)-enantiomer has sedative effects, whereas the (S)-isomer is teratogenic and can negatively influence the development of the embryo or foetus. Under biological conditions, the isomers interconvert, so separating the isomers before use is ineffective. As a result of this thalidomide disaster, many countries began to tighten drug approval regulations.
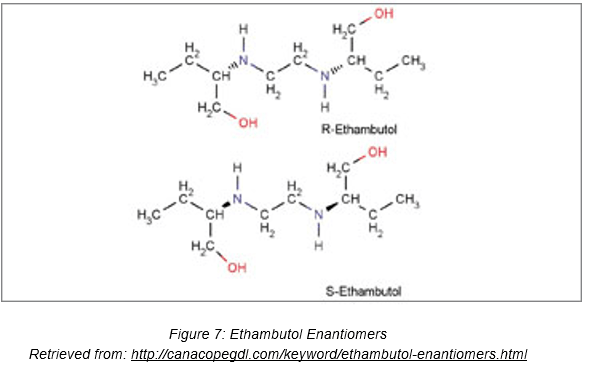
Here’s another drug that is useful, but can cause adverse effects too— Ethambutol. D-Ethambutol is an antituberculosis drug that can inhibit the transfer of mycolic acids into the cell wall of the tubercle bacillus [4]. It may also inhibit the synthesis of spermidine in mycobacteria. This decreases tubercle bacilli replication, thus preventing the spread of Mycobacterium tuberculosis in the human body. Nearly all strains of M. tuberculosis are sensitive to ethambutol. Given that tuberculosis is a potentially lethal infectious disease, Ethambutol sounds like an extremely beneficial drug, doesn’t it?
However, the other enantiomer of Ethambutol, L-ethambutol, has been found to cause blindness. Ethambutol-induced optic neuropathy (EON) is a well-known complication arising from the use of ethambutol, the severity of which is in a dose-dependent manner [4]. The toxicity is usually reversible upon discontinuation of Ethambutol, but there have also been several reports of permanent damage to the visual function. The clinical symptoms of EON include painless loss of central vision and partial alteration in the field of vision from the central vision to the natural blind spot.
As seen, the different enantiomers of drugs can have varied biological effects. It is not always safe to use racemic mixtures for drugs. This has an impact on the healthcare industry, as there is a need for regulations to ensure that both enantiomers of any chiral drug molecule are tested for biological activity including side-effects. Single enantiomers are less complex than racemic mixtures and hence seem to be better as adverse side reactions can be avoided. The preparation of enantiomerically pure drugs, and the resolution of racemate drugs, are, therefore, major areas of research today.
In conclusion, stereochemistry has offered grounds for the re-evaluation of current drugs in the market as well as new drugs that are being synthesized and is an important consideration in the pharmaceutical industry.
References
- Yartsev, A. Enantiomerism. 2018; Available from: https://derangedphysiology.com/main/cicm-primary-exam/required-reading/pharmaceutics/Chapter%202.0.1/enantiomerism.
- Chhabra, N., M.L. Aseri, and D. Padmanabhan, A review of drug isomerism and its significance. International journal of applied & basic medical research, 2013. 3(1): p. 16-18.
- Rivas, A. Thalidomide Birth Defect Victims Settle Class-Action Suit For $81 Million, Decades After Morning Sickness Pill Was Pulled Off The Market. 2013; Available from: https://www.medicaldaily.com/thalidomide-birth-defect-victims-settle-class-action-suit-81-million-decades-after-morning-sickness.
- Song, W. and S. Si, The rare ethambutol-induced optic neuropathy: A case-report and literature review. Medicine, 2017. 96(2): p. e5889-e5889.