So far, we have only been looking at applications of stereochemistry which is pretty manmade. However, mother nature seems to have some affinity for certain stereoisomers as well.
Let’s start by looking at the building blocks of proteins, amino acids. The general structure of amino acids can be given by this diagram, where X can be as simple as a hydrogen atom in the case of glycine or as complex as an indole in the case of tryptophan. Except for the case of glycine (which is prochiral anyway), all amino acids are chiral compounds since the carbon in red is a chiral centre. This means that these amino acids have enantiomers. However, all amino acids that are utilized in eukaryotic genetic structures are the l-stereoisomers, or ‘left-handed’ type, that is, these amino acids specifically have the structure shown on the right. While d-stereoisomers do of course exist, these amino acids never get picked by ribosomes during translation at all [1, 4, 5].
On the other hand, naturally occurring sugars tend to be the d-stereoisomers or the ‘right handed’ type. Glucose, for example, only occurs naturally as a d-glucose. l-glucose has to be synthesized in the laboratory. While both d-glucose and l-glucose taste pretty much the same, yet our body is specific and will only bind to d-glucose (and other d-hexose). As a result, if by some slim chance you are eating l-glucose, it will taste sweet as usual, but it will ‘pass right through’ and not be absorbed into the body [10, 18]. Too bad l-glucose is still too expensive to manufacture so we can’t have that as a substitute sweetener yet.
This property also extends to deoxyribose sugar that is a precursor of DNA (deoxyribonucleic acid). Deoxyribose sugar naturally occurs in the d–form. Combine this with the absolute stereochemistry of the nitrogenous bases, the nucleosides all have the R configuration about C1. This causes the double helical structure of the DNA to have be right handed in its normal state [8, 9].
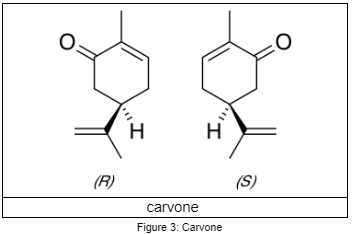
Another interesting ‘application’ of chirality in nature deals with tastes and smells. Interestingly, R-(–)-carvone smells similar to spearmint leaves while S-(+)-carvone smell similar to caraway seeds despite the similar structure. This is because the olfactory receptors are chiral. As a result, they respond differently to two enantiomers [12]. Another pair of enantiomers also exhibit this property. l-limonene is found in mint oil and smells like turpentine, while d-limonene is found in orange and, you guess it, smells like orange [10, 13].
References
- https://en.wikipedia.org/wiki/Amino_acid
- https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Chirality/Absolute_Configuration%2C_R-S_Sequence_Rules
- https://www.scripps.edu/baran/images/grpmtgpdf/OHara_Jun_12.pdf
- https://en.wikipedia.org/wiki/Nucleoside
- https://en.wikipedia.org/wiki/Nitrogenous_base
- https://link.springer.com/article/10.1007%2FBF01946717
- https://www.nature.com/articles/275159a0
- https://phys.org/news/2016-02-nature-mirrorthe-code-chirality.html
- https://en.wikipedia.org/wiki/Nucleic_acid_double_helix
- https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_05%3A_Stereochemistry_at_Tetrahedral_Centers/5.12%3A_Chirality_in_Nature_and_Chiral_Environments
- https://pharmaceuticalintelligence.com/2016/03/15/l-form-chirality-of-dna-nucleotides/
- https://en.wikipedia.org/wiki/Olfactory_receptor
- https://en.wikipedia.org/wiki/Limonene
- https://smellofevolution.com/2014/11/26/the-chirality-of-smell/
- https://pubs.acs.org/doi/abs/10.1021/bk-2015-1212.ch013
- https://en.wikipedia.org/wiki/L-Glucose
- https://phys.org/news/2016-06-riddle-life-single-handedness.html
- https://www.the-scientist.com/news/left-handed-sugar-gets-a-free-ride-out-of-your-system-60471
- https://www.masterorganicchemistry.com/2017/05/24/d-and-l-sugars/
- https://en.wikipedia.org/wiki/Chirality_(chemistry)
- https://www.forbes.com/sites/brucedorminey/2013/06/29/lifes-left-handed-amino-acids-remain-astrobiological-head-scratcher/#20f7cbd13b6d
- https://bio.libretexts.org/TextMaps/Biochemistry/Book%3A_Biochemistry_Online_(Jakubowski)/02%3A_PROTEIN_STRUCTURE/2A%3A_Amino_Acids/A02._Amino_Acid_Stereochemistry
- https://www.ks.uiuc.edu/Training/Tutorials/science/structurecheck/tutorial_structurecheck-html/node3.html
- https://www.thoughtco.com/amino-acid-chirality-4009939
- https://www.smithsonianmag.com/space/must-all-molecules-life-be-left-handed-or-right-handed-180959956/
- https://en.wikipedia.org/wiki/Deoxyribose