In the first post, we have introduced you to the concept of cis-trans and E-Z isomerism but have not gone into the details. So, the time has come for us to dig into this.
Cis-trans Convention
The cis-trans convention is quite an intuitive one. In organic chemistry, it is generally used for disubstituted alkenes and alicyclic compounds. All you have to do is to figure out whether the two identical substituent groups are on the same side or different sides of the ring or carbon-carbon double bond. If the two are on the same side, it’s cis. If the two are on opposite sides, it’s trans. It’s that simple. An easy way to remember this convention is to look at the third letter of the word, for CIS the S stands for Same and for TRANS the A stands for Alternate.
For example, let’s look at 1,2-dichlorocyclohexane. We can have the two chlorine atoms on the same side of the ring hence the cis label, or we can have the two chlorine atoms on the opposite sides hence the trans label.
The same rule applies to alkenes as shown below.
E-Z Convention
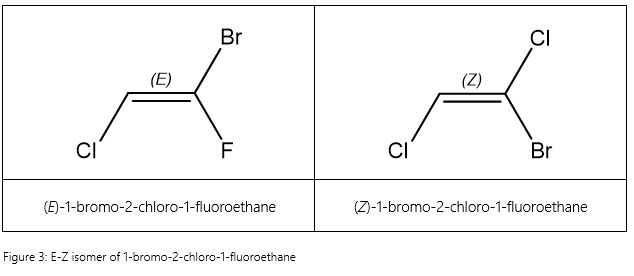
However, the issue with the cis-trans convention is that it only works unambiguously when there are at least one pair of identical substituent groups on the carbons. If you look at 1-bromo-2-chloro-1-fluoroethane, the cis-trans system no longer works properly. This is because the cis-trans system uses relative stereochemistry between two atoms. If we want to continue using the cis-trans system for tri-substituted molecules, we will end up having to specify the two atoms we are referring to. Of course, chaos will ensue in larger molecules, making the nomenclature difficult to read. Hence, we introduce another system based on absolute stereochemistry called the E-Z convention.
In the E-Z convention, we only look at the two substituents with the highest priority according to the Cahn–Ingold–Prelog (CIP) sequence rules. In both molecules (a) and (b), the two highest priority substituents are bromine and chlorine. In molecule (a), these two are opposite each other, hence we say that they are in an E configuration, which stands for ‘entgegen’ (‘opposite’ in German). In molecule (b), these two are on the same side, hence we say that they are in an Z configuration, which stands for ‘zusammen’ (‘together’ in German) [1]. EaZy, isn’t it?
Chemical Properties
While the chemical properties of cis-trans isomers cannot be easily predicted in general, for disubstituted alkenes, there are some general trends that we can deduce. First, in cis configuration, the dipole moments due to the substituents tend to add up, while in trans configuration, the dipole moments due to the substituents tend to cancel each other out. As a result, cis molecules tend to be more polar than trans molecule. Hence, cis molecules also generally have higher boiling points than that of their respective trans counterparts. however, in the case of larger substituents, trans alkenes tend to be more symmetrical than their cis counterparts, allowing them to be better packed in the solid state Hence, trans alkenes have higher melting points [2].
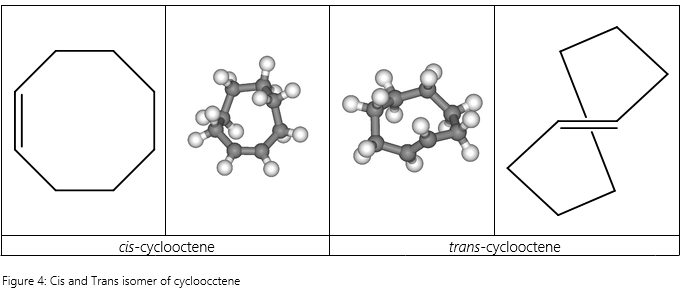
Another interesting property of cis–trans isomers lies in the stability of cycloalkenes versus that of acyclic alkenes. In acyclic alkenes, the trans isomers tend to be more stable than the cis isomers due to reduced steric hindrance. However, in cyclic alkenes with less than 8 carbons, you may notice that we almost always draw them exclusively in the cis configuration. This is because the trans isomers tend to be very much less stable than the cis isomers due to twisting of the double bond. An example would be to look at cyclooctene [3].
Very clearly, we can see that the trans configuration on just one double bond causes extreme twisting of the ring structure which deviates greatly from the usual sp2 angles. In other words, the angular strain is very large in the trans configuration, causing the trans cycloalkenes to be less stable than their cis counterparts for smaller cycloalkenes.
However, in cyclic alkenes with more than 11 carbons, the trans isomer is more stable than the cis isomer as the ring strain is less significant in a bigger ring and the destabilizing effects of steric hindrance in the cis-state outweighs the ring strain in the trans state.
Taking cyclohexadecene, a 16-carbon cycloalkane, as an example, the cis configuration sees many hydrogen atoms crowding very near one another and quite a number right behind each other. On the other hand, while the trans configuration does not manage to achieve the ideal angle, the steric and torsional strains are much lower than the cis configuration, making it the more stable form.
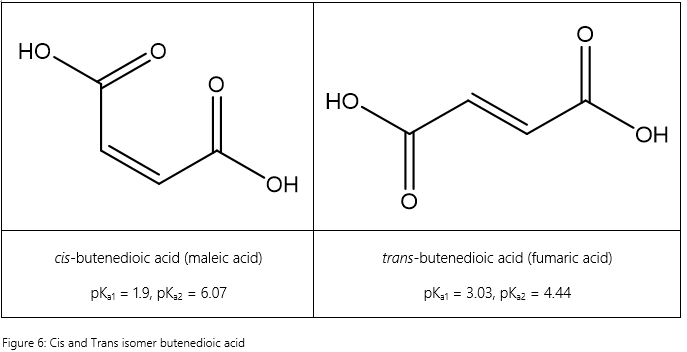
The case of butenedioic acid is also an interesting one. Butenedioic exists in two forms as shown below.
The cis isomer, maleic acid, donates its first proton much more easily than the trans isomer, fumaric acid. This is due to the geometry of maleic acid which allows intramolecular hydrogen bonding to stabilize the conjugate base as the –OH groups can be in close proximity with one another, a property which fumaric acid lacks due to its geometry. However, also due to the intramolecular hydrogen bond, the hydrogen in the conjugate base of maleic acid experiences a stronger attraction to the molecule (2 O—H instead of 1 O-H) and thus fumaric acid deprotonates the second hydrogen much more easily than the maleic acid as well.
References
- Burrows, A., Holman, J., Parsons, A., Piling, G., and Price, G. (2017) Isomerism and stereochemistry, in Chemistry³: introducing inorganic, organic and physical chemistry3rd ed. essayOxford University PressOxford.
- (2018, June 11) Cis–trans isomerism. Wikipedia. Wikimedia Foundation.
- Höfker, U., and Fels, G. Stereoisomers of Cyclic Compounds. Chemgapedia.